History and Arrangement of the Periodic Table Unit

History and Arrangement of the Periodic Table Unit 4 Day 1

Content Objective � I CAN explain the historical development of the Periodic Table and use the Periodic Table to identify and explain the properties of chemical families, including alkali metals, alkaline earth metals, halogens, noble gases, and transition metals

Criteria for Success I CAN explain the contributions of Mendeleev and Mosley to development of the periodic table. � I CAN explain how the Periodic Table was originally organized and compare the original organization to how it is organized today � I CAN explain how the electron configurations of the elements contribute to their organization and properties on the periodic table �
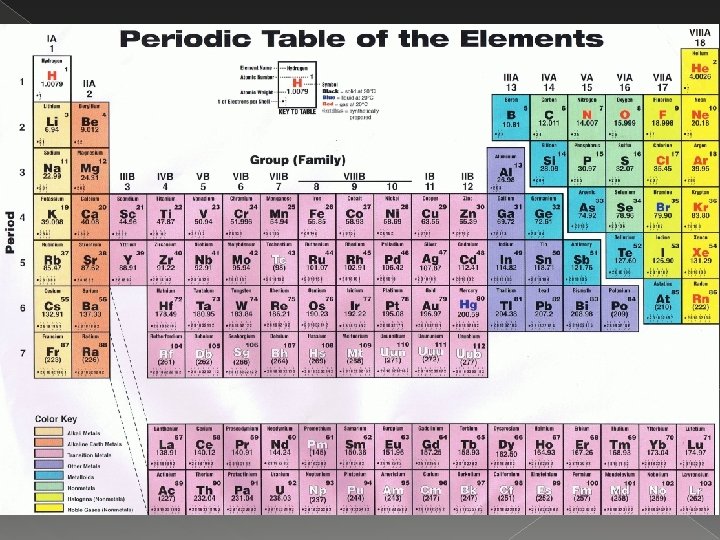
History of the Periodic Table � A. Russian chemist, Dmitri Mendeleev __________, originally organized mass He the periodic table by atomic _____. was able to predict missing elements ________________

History of the Periodic Table Moseley Henry _______ helped to develop our modern periodic table arranged by mass atomic _____. � B.
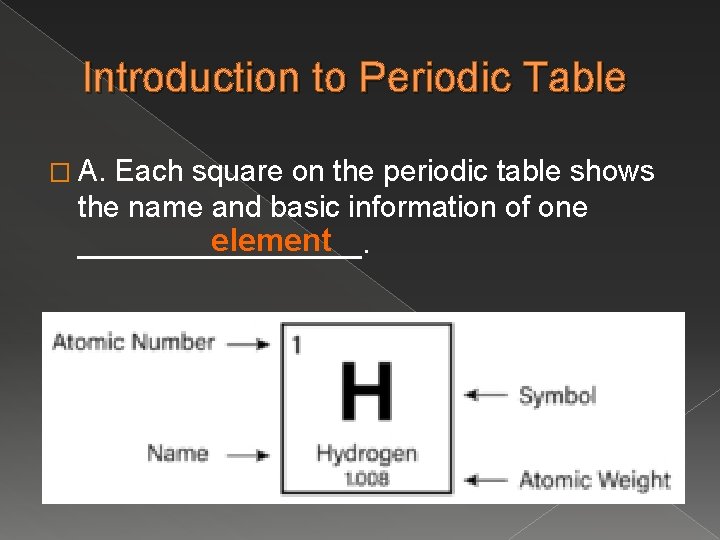
Introduction to Periodic Table � A. Each square on the periodic table shows the name and basic information of one element _________.
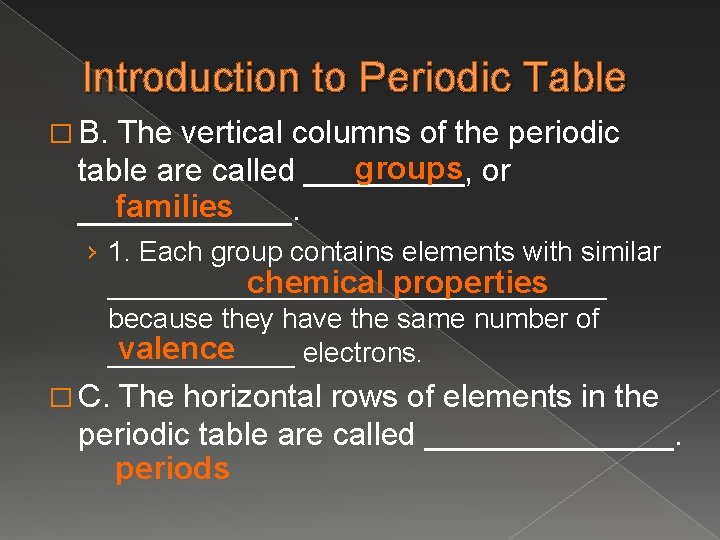
Introduction to Periodic Table � B. The vertical columns of the periodic groups or table are called _____, families ______. › 1. Each group contains elements with similar chemical properties ________________ because they have the same number of valence ______ electrons. � C. The horizontal rows of elements in the periodic table are called _______. periods

Types of Elements metal A ______ is an element that good is a _____ conductor of heat and electricity. › 1. Most metals are also � A. �Lustrous Malleable �_______ ductile �and _______
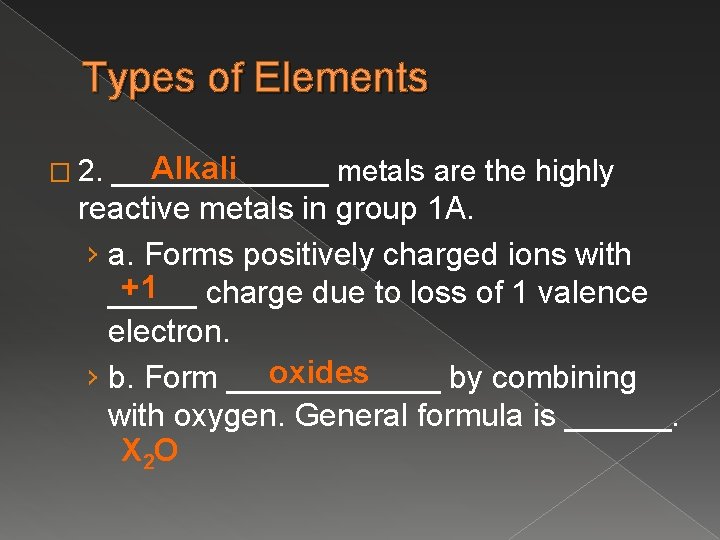
Types of Elements � 2. Alkali _______ metals are the highly reactive metals in group 1 A. › a. Forms positively charged ions with +1 _____ charge due to loss of 1 valence electron. oxides › b. Form ______ by combining with oxygen. General formula is ______. X 2 O
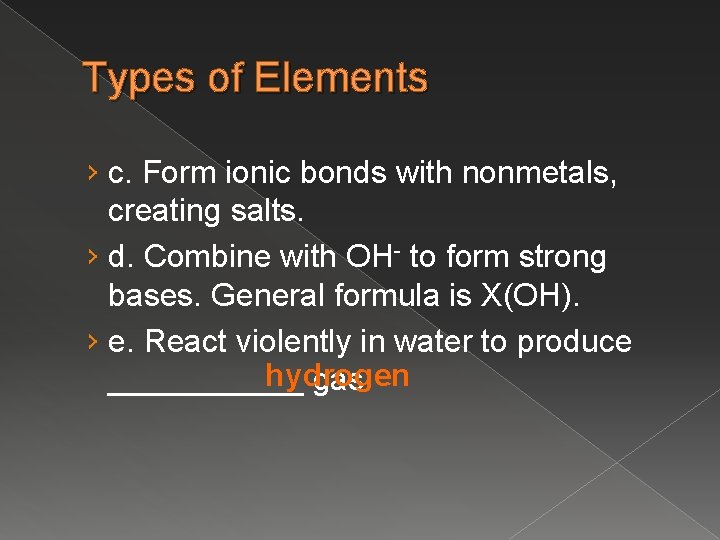
Types of Elements › c. Form ionic bonds with nonmetals, creating salts. › d. Combine with OH- to form strong bases. General formula is X(OH). › e. React violently in water to produce hydrogen ______ gas.


Types of Elements Alkaline earth _____________ metals are the metals in group 2 A. � 3. › a. Forms positively charged ions with _____ +2 due to loss of 2 valence electrons. charge › b. Form _______ by combining with oxides oxygen. General formula is ______. XO
Types of Elements › c. Form ionic bonds with nonmetals, creating salts. › d. Combine with OH- to form strong bases. General formula is X(OH)2.

Types of Elements Transition _________ metals are between groups 2 A and 3 A. � 4.
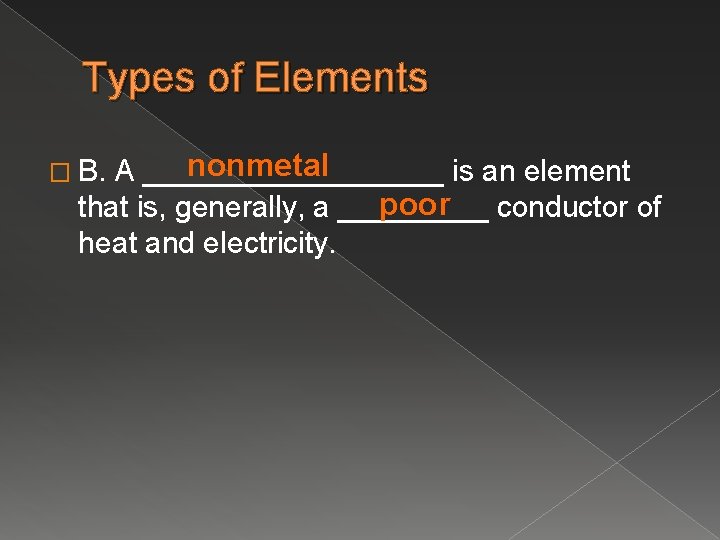
Types of Elements nonmetal A _________ is an element poor conductor of that is, generally, a _____ heat and electricity. � B.

Types of Elements › 1. Most non-metal solids tend to be brittle _______ rather than malleable and ductile.

Types of Elements › 2. The Group 17 elements are also known as halogens and are extremely the ________ reactive. › 3. The Group 18 elements are also known as noble gases the __________ and are relatively unreactive, or inert.
Types of Elements metalloid A ________ is classified as an element that has some characteristics of both metals and nonmetals. � C. › 1. Metalloids tend to be semiconductors ___________ of electricity.
Types of Elements � D. Seven elements on the periodic table diatomic state: H 2, exist in the ________ N 2, O 2, F 2, Cl 2, Br 2, and I 2. › 1. Use the mnemonic: �Have No Fear Of Ice Cold Beverages �Br. INCl. HOF
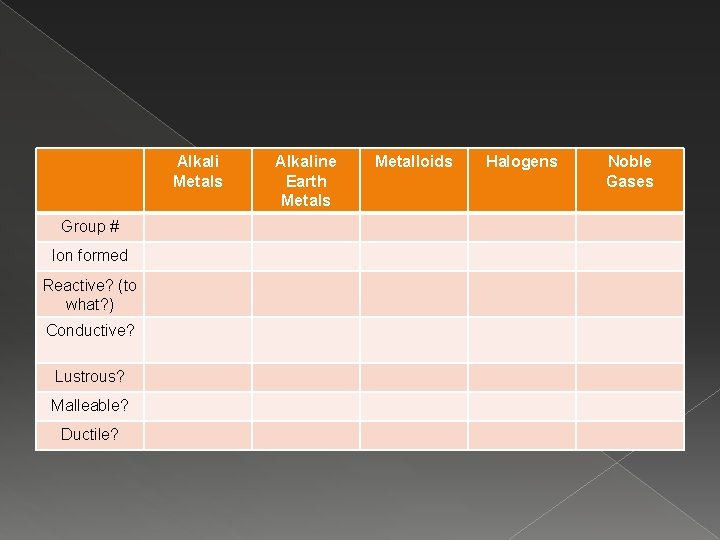
Alkali Metals Group # Ion formed Reactive? (to what? ) Conductive? Lustrous? Malleable? Ductile? Alkaline Earth Metals Metalloids Halogens Noble Gases
- Slides: 30