Hinge Point Questions Hi I hope you find

















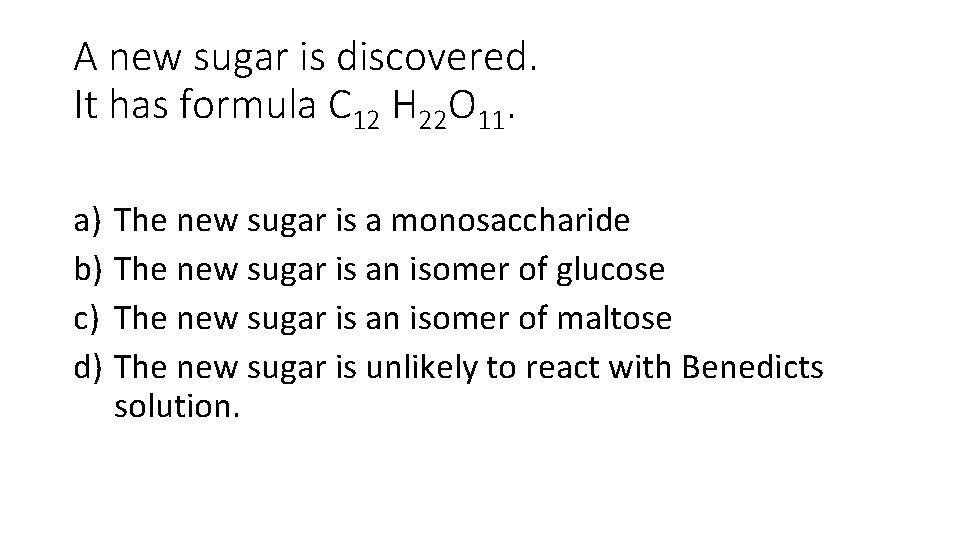




- Slides: 30

Hinge Point Questions

Hi, I hope you find these hinge point questions useful. They are aimed at National 5 Chemistry in Scotland, which is about the same level as KS 4 or GCSE. Please feel free to adapt them for your own use. I have written them for the areas I feel my pupils struggle with most. Some are straightforward one right answer, others have more than one correct answer, while others are aimed to promote ideas and discussion.

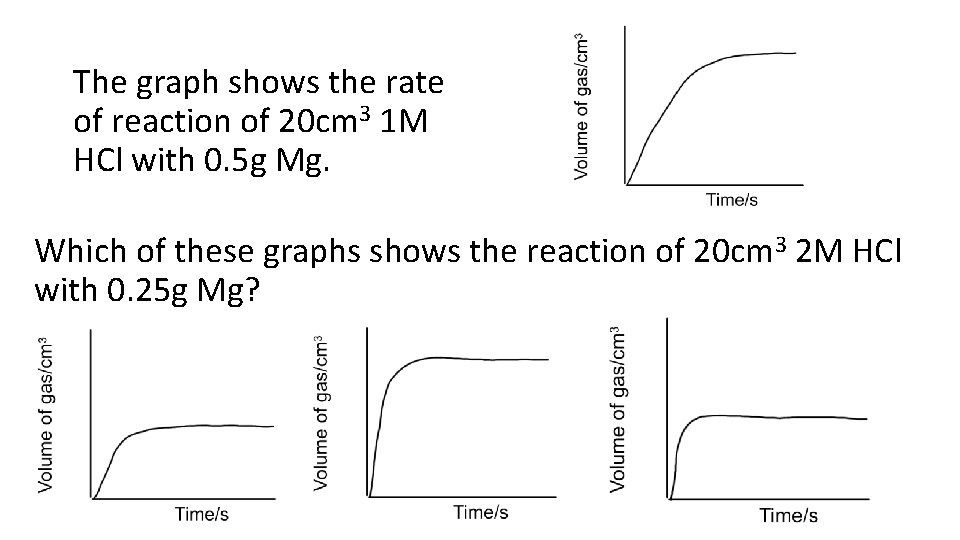
The graph shows the rate of reaction of 20 cm 3 1 M HCl with 0. 5 g Mg. Which of these graphs shows the reaction of 20 cm 3 2 M HCl with 0. 25 g Mg?

Which of these is the correct formula for ammonium sulphate? • A) (NH 3)2 SO 4 • B) (NH 4)2 SO 4 • C) NH 3 SO 4 • D) (NH 4)(SO 4)2

A teacher asks the class to describe why atoms are neutral. Which answer is best? a) The particles in the atom cancel out. b) Atoms have protons and electrons in the nucleus, which cancel out. c) The neutrons make them neutral. d) Atoms have the same number of protons and electrons which cancel out.

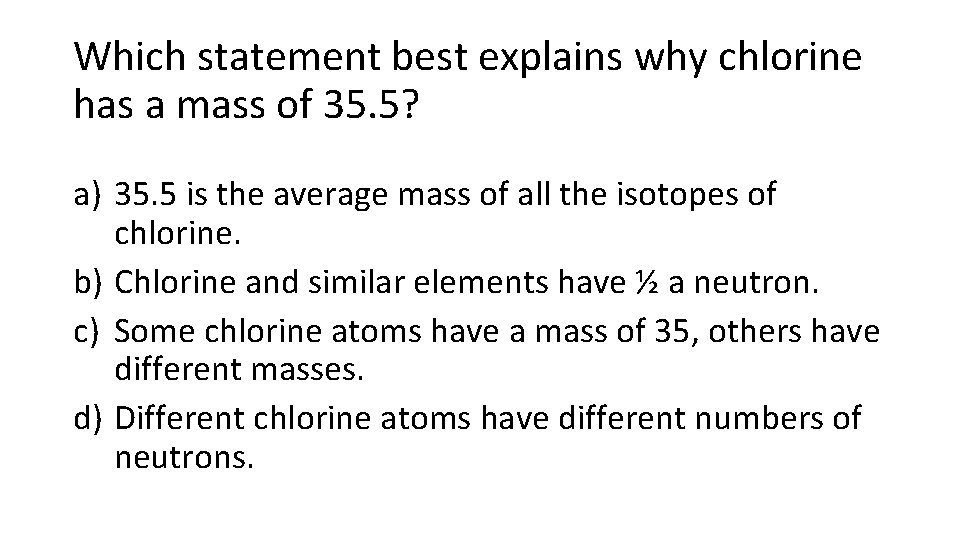
Which statement best explains why chlorine has a mass of 35. 5? a) 35. 5 is the average mass of all the isotopes of chlorine. b) Chlorine and similar elements have ½ a neutron. c) Some chlorine atoms have a mass of 35, others have different masses. d) Different chlorine atoms have different numbers of neutrons.

If a substance conducts electricity, what type of bonding does it have?

Which statement best explains why ions form? a) Metals form positive ions because they lose electrons. b) Positive ions form because loss of electrons leads to a stable electron arrangement. c) Ions are more reactive than atoms. d) Ionic bonding is stronger than London forces.
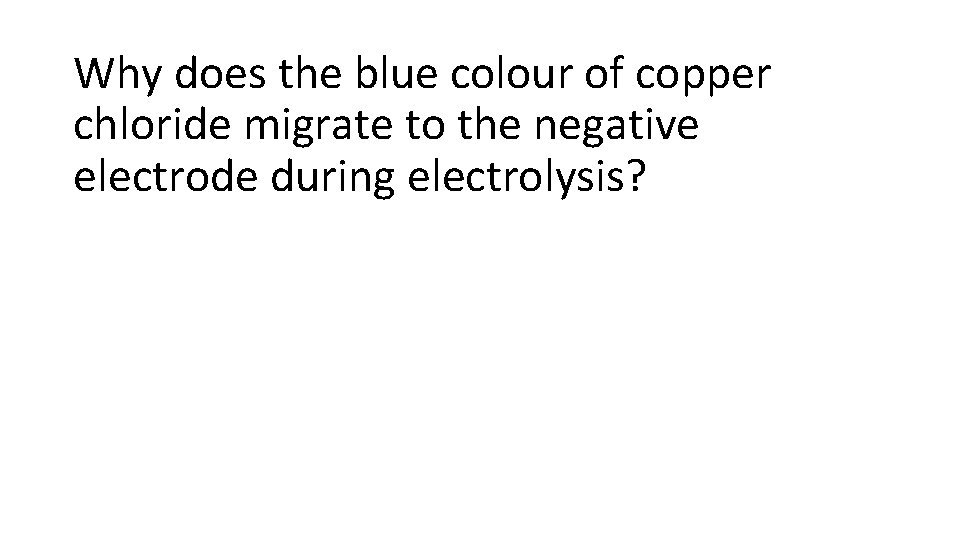
Why does the blue colour of copper chloride migrate to the negative electrode during electrolysis?

As you go down the fractionating column… a) flammability, viscosity and ease of evaporation decrease b) Flammability and viscosity decrease, ease of evaporation increases. c) Flammability and ease of evaporation increase, viscosity decreases. d) Flammability and ease of evaporation decrease, viscosity increases.

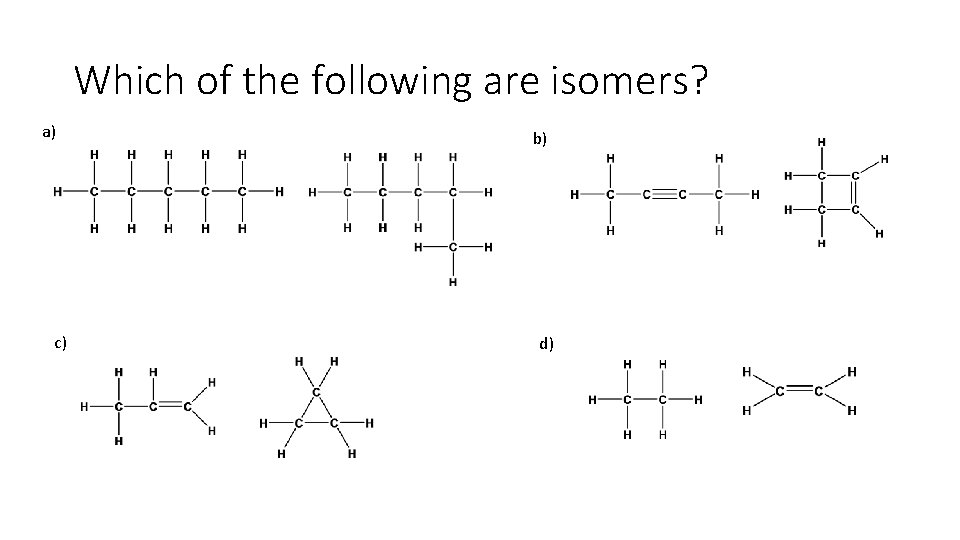
Which of the following are isomers? a) c) b) d)

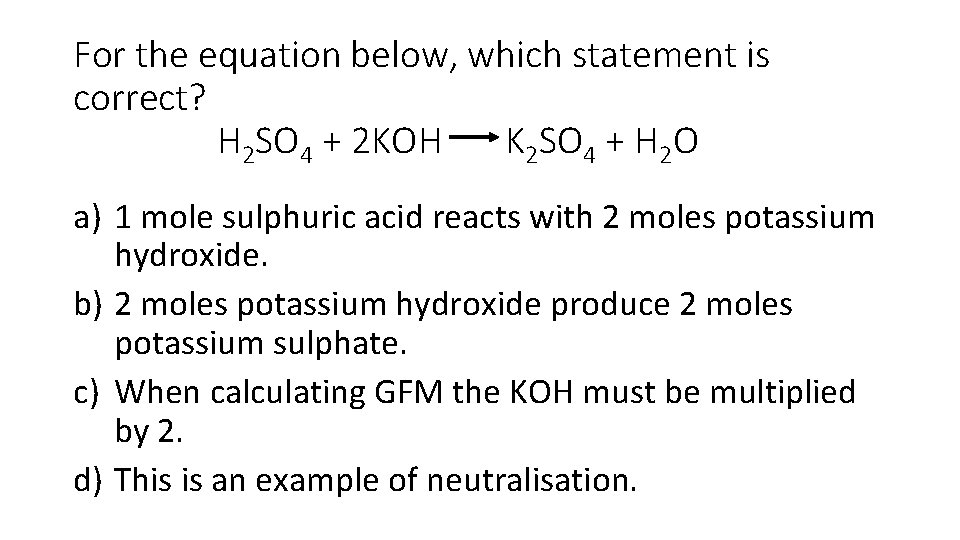
For the equation below, which statement is correct? H 2 SO 4 + 2 KOH K 2 SO 4 + H 2 O a) 1 mole sulphuric acid reacts with 2 moles potassium hydroxide. b) 2 moles potassium hydroxide produce 2 moles potassium sulphate. c) When calculating GFM the KOH must be multiplied by 2. d) This is an example of neutralisation.

For a titration, rank these statements in order of importance. a) Reading the bottom of the meniscus on the burette b) carrying out a rough titration first c) Ensuring concordancy d) Clean glassware

Which is correct for an alkaline solution? a) The concentration of H+ ions is greater than OH- ions. b) [OH-] > 10 -7 c) p. H increases when water is added. d) Ammonia solution dissociates fully in water.

A pupil tested the properties of lithium oxide. Which statement or statements match what they found? a) Has no effect on p. H. b) Produces an alkali if dissolved in water. c) Produces an acid if dissolved in water. d) Conducts electricity when molten.

Which of these is the correct ionic formula for sodium sulphate? 1. Na 2+SO 42+ 22. (Na )2 SO 4 3. Na+SO 424. Na. SO 4

A metal X reacts with dilute acids and is extracted from its ore by electrolysis. It is not stored under oil. a) X can be displaced from a solution of its ions by copper b) A solution of X reacts with sodium metal to precipitate out X. c) Heating the oxide of X with carbon produces metallic X. d) X can be found uncombined.

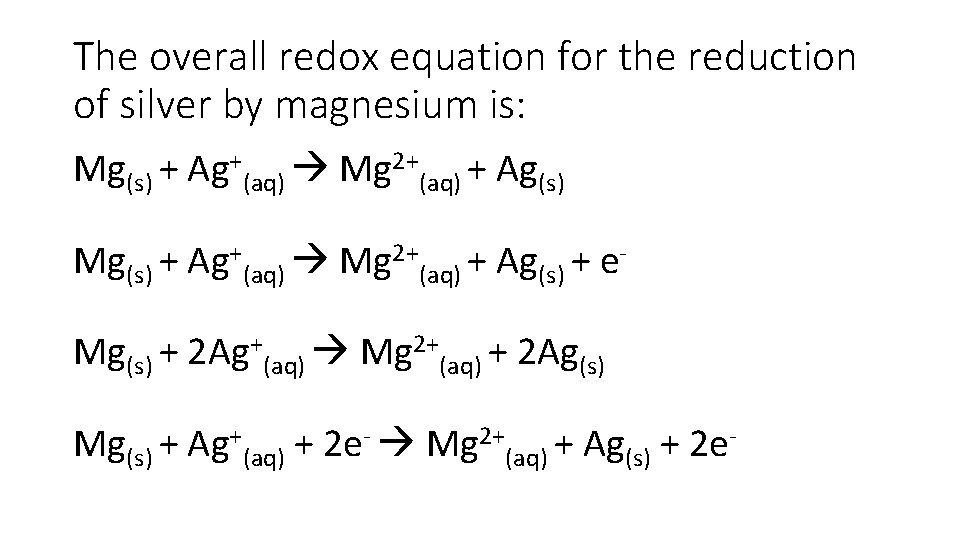
The overall redox equation for the reduction of silver by magnesium is: Mg(s) + Ag+(aq) Mg 2+(aq) + Ag(s) + e. Mg(s) + 2 Ag+(aq) Mg 2+(aq) + 2 Ag(s) Mg(s) + Ag+(aq) + 2 e- Mg 2+(aq) + Ag(s) + 2 e-

The Queensferry crossing in Edinburgh was built using steel caissons, large watertight tanks used to create the bridge towers under the water. To protect the workers inside them, the caissons must remain structurally sound and not rust. Discuss which method of protection against corrosion would be most suited to protecting the caissons.

Which of these electrochemical cells would produce the highest voltage and be a practical consumer option? a) lithium-gold b) Potassium-copper c) Lead-copper d) Aluminium-tin

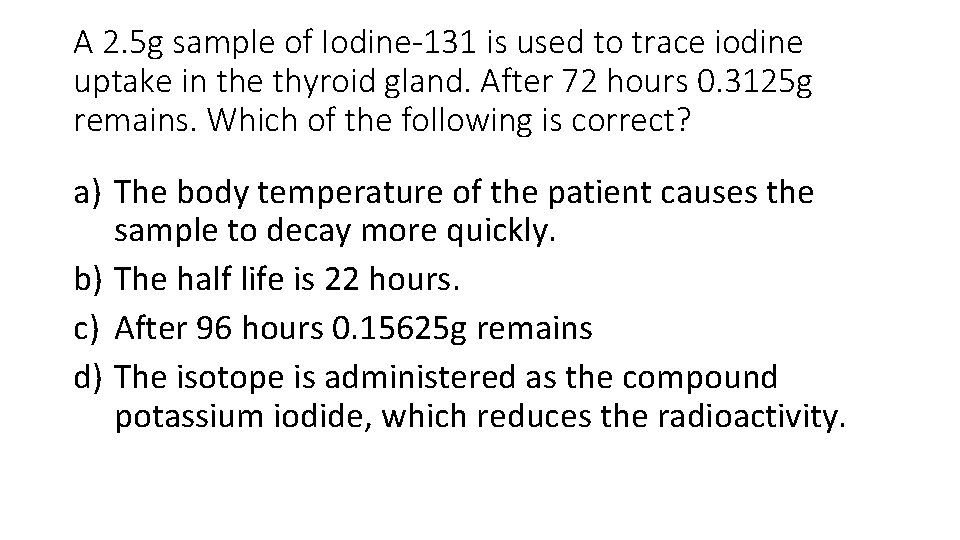
A 2. 5 g sample of Iodine-131 is used to trace iodine uptake in the thyroid gland. After 72 hours 0. 3125 g remains. Which of the following is correct? a) The body temperature of the patient causes the sample to decay more quickly. b) The half life is 22 hours. c) After 96 hours 0. 15625 g remains d) The isotope is administered as the compound potassium iodide, which reduces the radioactivity.

The correct name for the functional group of the alkanols is: a) Carbonyl b) Alcohol c) Hydryl d) hydroxyl

Alkanoic acids differ in their reactions with mineral acids such as HCl. Which statement is most accurate? a) 10 cm 3 of a 0. 1 mol l-1 solution of ethanoic acid needs the same volume of 0. 1 mol l-1 Na. OH to neutralise compared with 10 cm 3 of a 0. 1 mol l-1 solution of HCl. b) Propanoic acid reacts more quickly with zinc than sulphuric acid. c) Ethanoic acid dissociates into ions. d) The p. H values of ethanoic acid and nitric acid are similar.

Which is these statements about esters is true? a) They can be used for flavourings and perfumes. b) They are made from carboxylic acids and alkenes. c) They are made from alkanols and carboxylic acids. d) They are formed by addition reactions.
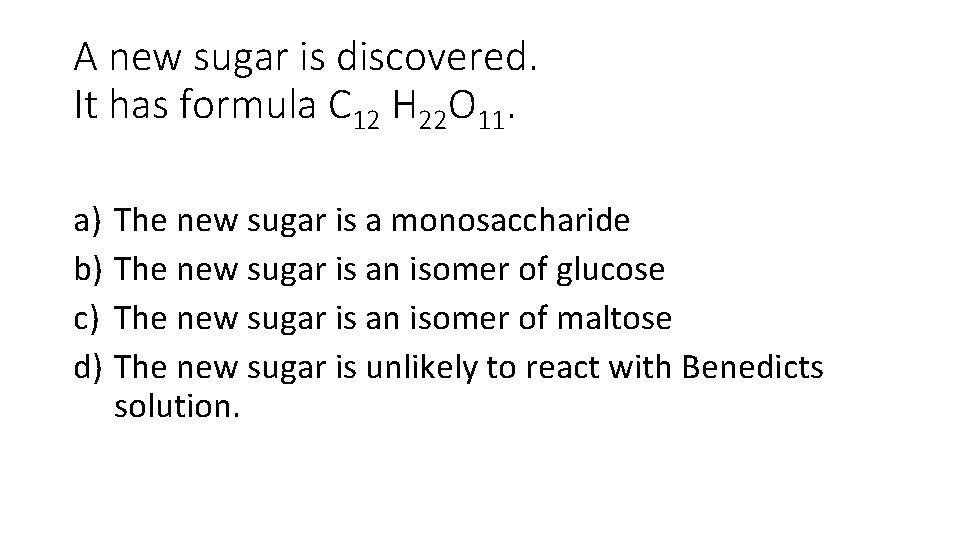
A new sugar is discovered. It has formula C 12 H 22 O 11. a) The new sugar is a monosaccharide b) The new sugar is an isomer of glucose c) The new sugar is an isomer of maltose d) The new sugar is unlikely to react with Benedicts solution.

Which statement best explains the food vs fuel dilemma? a) Land is needed to grow food crops to feed a population, but a developing society also needs to grow biofuel crops to produce energy to help them develop. They cannot do both. b) Some crops grow better than others. c) Food crops need more fertiliser so it is better and cheaper to grow biofuel crops. d) Biofuel crops need more fertiliser so it is better and cheaper to grow food crops.

Which of these is correct: a) The Haber process uses a Pt catalyst to make ammonia. b) The Ostwald process uses an Fe catalyst to make ammonia. c) The Haber process produces a large yield. d) The Ostwald process is exothermic.

Which of the following compounds would be most suitable as a fast acting fertiliser? a) Sodium nitrate b) Potassium nitrate c) Ammonium nitrate d) Calcium phosphate

Which of the following is correct? a) Proteins are examples of addition polymers. b) Proteins only come from animal sources. c) Proteins are made from monomers called amino acids. d) Adding Na. OH to a protein solution produces an alkaline gas.

Oils can be made into spreadable butter. Which of the following statements is correct? a) The process is called hydrogenation. b) The process is called addition. c) The butter has a higher degree of unsaturation than the oil. d) The chemical formula of the oil would have a C: H ratio of Cn: H 2 n+2