HighLevel Disinfection Jennifer Zeck RN BSN CRCST Objectives

High-Level Disinfection Jennifer Zeck, RN, BSN, CRCST

Objectives • • • Review current recommendations and standards for best practices in reprocessing instruments, endoscopes, probes, and other reusable equipment Review current recommendations and standards for quality assurance, monitoring, and documentation of the disinfection process Evaluate the training and competency process Identify tools and resources to support audits and training Identify areas of risk based on literature and internal audits 6/5/20 21 2
Classification of Instruments/Equipment E. H. Spaulding believed that how an object will be disinfected depended on the object’s intended use ***This classification system is being examined due to outbreak issues*** Critical: objects that enter sterile body sites/tissues Ex: surgical stainless steel Semi-critical: objects that touch mucous membranes Ex: endoscopes, laryngoscopes, probes Non-critical: objects that touch only intact skin Ex: BP cuffs, stethoscopes, blood glucose monitors 6/5/20 21 3
Processing Semi-Critical Patient Care Items Method: High-level disinfection (HLD) (kills all microorganisms, except high numbers of bacterial spores) Options: Automated Endoscope Reprocessors (AERs), Trophon, manual soaking Key points: Use of sheaths does not eliminate need for HLD after each use Proper cleaning is essential for HLD to be effective 6/5/20 21 4

Important Safety Considerations Wear appropriate PPE when working with highlevel disinfectants (e. g. , right type of gloves, protective eyewear, masks, gowns or aprons) Read the material safety data sheet for the product and ensure room meets safety requirements (e. g. , ventilation) Eye wash stations Chemical bins should be tightly covered when not in use 6/5/20 21 5

Endoscope Reprocessing Steps Pre-Cleaning Transport to reprocessing area Leak-Testing Manual Cleaning High-Level Disinfection (HLD) Rinsing Drying Storage Documentation 6/5/2021 6

Disinfection Begins at Point of Use! Pre-clean: at point-of-use, remove debris by wiping exterior and aspirating detergent through air/water and biopsy channels. Follow manufacturer’s IFU & ensure all steps complete! 6/5/2021 7

Disinfection Begins at Pointof-Use Pre-cleaning: • Prevents biofilm development • Protects scopes and probes • Facilitates decontamination and cleaning • Inspect for damage • Use approved enzymatic detergent 6/5/20 21 8

Transport of Soiled Scopes/Probes Bins with lids Impermeable color bags Closed/covered Closed carts container systems Biohazard label Transport as soon as possible Protect the scope/probe! $$$ 6/5/20 21 9

Leak Testing Detects damage to the scope that would otherwise allow fluid to invade areas of the scope that cannot tolerate fluid. Must be done before EVERY reprocessing cycle. Follow Wet IFU’s vs. Dry Allow enough time to perform a thorough test= Saving $$$ 6/5/2021 10

Manual Cleaning Ensure physical space adequate Mechanically clean with water and enzymatic detergent. Thoroughly brush and flush and rinse according to IFU Depending on the type of scope/probe, may include many steps Olympus Duodenoscope has 139 manual cleaning steps! One set of supplies for each scope. Unresolved topic regarding skipping some cleaning steps if allowed by scope and AER IFU’s 6/5/2021 11
High-Level Disinfection (HLD) Immerse scope/probe and perfuse HLD/ through all channels for exposure time and temp per IFU If automatic endoscope preprocessor (AER) used, review model-specific reprocessing protocols from both the endoscope and automatic endoscope preprocessor (AER) manufacturer 6/5/2021 12
HLD Parameters Temperature: must meet parameters set by disinfectant IFU - must be monitored Life cycle: disinfectant should be used only for the life span defined by the IFU, or if MIC test fails QA test: HLD should be testing prior to each use to ensure disinfectant is effective Disposal: may be made inert prior to disposal depending on chemical. Check with local municipality requirements Proper PPE and environmental considerations are critical 6/5/20 21 13

Rinsing Thoroughly flush and rinse with sterile or filtered tap water; flush channels with alcohol AER’s perform this step Ensure water quality matches IFU 6/5/2021 14

Drying AER’s often have this step but may not be sufficient Critical step which if often underperformed Current studies show this may take up to 10 -minutes of forced, instrument grade air to sufficiently dry inner lumens 6/5/2021 15
Storage Hang in vertical position with all controls in unlocked position Accessories should be stored OFF the scope Cabinet should protect scope from environment HEPA Hang filtered vs. standard time= unresolved issue 6/5/2021 16
Documentation Must be able to trace scopes to each patient use and include critical data points Date/Time Soak/exposure time to the disinfectant Temperature of the disinfectant Results of quality tests (MEC results) Name of the patient the item was used on The transducer serial number or tracking number Name of the employee performing the HLD process Expiration date and lot number of the solution test strips Expiration date and lot number of the disinfectant Newer data points to consider: End of case time Beginning of cleaning time 6/5/2021 17

Auditing/Tracing Regularly audit the adherence to all reprocessing steps, from pre-cleaning to storage Check for current manufacturers instructions and training and competency records Monitor automated endoscopic reprocessing performance (AER), including QA’s 6/5/20 21 18

Auditing/Tracing cont’d. Should be conducted in all areas where reprocessing occurs Can be very difficult in large facilities Know where reprocessing is occurring Thorough review of all clinical areas should occur Provide feedback to personnel/department managers on audit findings Ensure facility follows their own policies, and that practice matches policy 6/5/20 21 19

Training and Competency Training should be performed Upon hire or prior to taking on reprocessing responsibilities Annually By and when new devices are introduced fully competent person Competency Should be verified by return demonstration Must be verified prior to performing reprocessing independently Records must be maintained for each employee 6/5/20 21 20

Training and Competency cont’d. Manufacturer Instructions for Use (IFU) Must be available and current for all instruments/equipment Guidelines: ST 91, SGNA, AORN, CDC, Internal Risk Assessment 6/5/2021 21

CMS HLD Hot Topics Semi-critical equipment is HLD or sterilized Items are pre-cleaned according to manufacturer’s instructions prior to HLD Medical devices and instruments are visually inspected for residual soil and re-cleaned as needed before HLD According to manufacturer instructions, chemicals used for high-level disinfection are Prepared Tested for appropriate concentration Replaced Documented to have been prepared and replaced 6/5/20 21 22

CMS HLD Hot Topics cont’d. Instruments requiring high-level disinfection are Disinfected for the appropriate length of time as specified by manufacturer’s instructions Disinfected at the appropriate temperature as specified by manufacturer’s instructions Items that undergo HLD are allowed to dry before use Following HLD, items are stored in a designated clean area in a manner to prevent contamination 6/5/20 21 23

Areas of Risk ERCP/Duodenoscope outbreaks Ureteroscopes Bronchoscopes Simethicone Ultrasound probes 6/5/2021 24
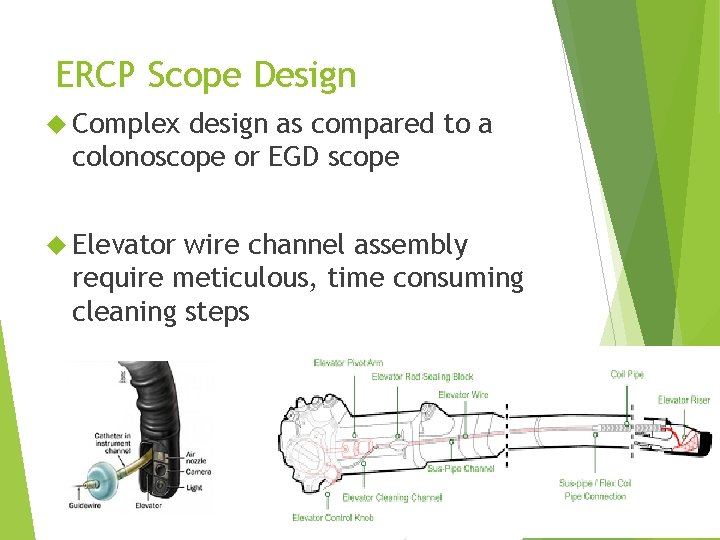
ERCP Scope Design Complex design as compared to a colonoscope or EGD scope Elevator wire channel assembly require meticulous, time consuming cleaning steps 6/5/2021 25

CRE Outbreaks Related to Contaminated ERCP Scopes Began as early as April 2009 in Florida. Received little attention Late 2011 -2013: Multiple outbreaks traced to duodenoscopes (ERCP scopes), despite following FDA approved manufacturer cleaning instructions 2013: CDC advises FDA that it may not be possible to disinfect duodenoscopes following FDA approved IFU’s FDA begins reviewing 2014 -2015: CRE outbreaks continue be reported, including many high profile medical centers February 2015: FDA issues first specific warning related to duodenoscopes, even when all 6/5/2021 26 manufacturer cleaning instructions are followed

2014 UCLA Outbreak Index patient, awaiting a liver transplant, underwent ERCP on Oct 3 § She was unknowingly carrying CRE Two weeks later, 18 year old admitted with acute pancreatitis, receiving an ERCP 4 days later § § Within days he became septic, placed on vent Physicians were baffled as to how he contracted CRE 6/5/2021 27

2014 UCLA Outbreak cont’d. 40 year old healthy woman became septic following routine ERCP for gallstone removal § Her doctor began looking into how she could have got CRE 18 year old patient discharged after 83 days, and returned a few weeks later for repeat ERCP with stent removal § He again crashed and became septic 6/5/2021 28

Connecting the Dots… UCLA microbiologists confirm matching CRE among several patients Later that day confirmed scopes as source of CRE § Scopes were only 7 months old and had been cleaned per IFU’s § 6/5/2021 29

Aftermath 8 patients contracted CRE infection, with 3 fatalities Soon after, FDA issues safety alert to all US hospitals In March, Olympus provides new cleaning instructions, along with a new cleaning brush. 2013 Olympus warned European hospitals of the risk of CRE in duodenoscopes, but did not in the US 6/5/2021 30

Interim Duodenoscope Surveillance Protocol Guidelines issues by CDC in response to reports that properly reprocessed endoscopes may be contaminated with CRE § Surveillance culturing of duodenoscopes § Remedial actions § Patient Notification § Training and competency 6/5/2021 31

Surveillance Culturing CDC established protocol for performing cultures of duodenoscopes Not required To provide guidance for facilities wishing to do surveillance No established frequency Samples from channel and distal tip, around elevator After reprocessing Monitor for high-concern organisms, such as gram negative bacteria, not commonly found contaminants of low concern 6/5/2021 32

Remedial Actions Reprocess again, with repeat culturing Review processes Consider having manufacturer evaluate the scope Search for clusters among patients 6/5/2021 33

Ofstead’s findings for ureteroscopes that had undergone cleaning and sterilization (N=16) 100% had visible residue or defects 100% had substantial protein 63% had detectable hemoglobin 44% had ATP above background 13% had microbial growth Sterilization won’t work if it’s not clean! Ofstead, AJIC 2017

Bronchoscope reprocessing study Prospective, observational study conducted in 2017 Sites: 3 large multi-specialty hospitals in the US Non-enzymatic detergent Medivators Advantage Plus AERs with peracetic acid HLD Subjects: 24 clinically-used and 2 new bronchoscopes Assessments (in the order performed): Tests for residual contamination after cleaning and after HLD Visual examinations Observation of reprocessing activities Evaluation of storage cabinet cleanliness © Ofstead & Associates, Inc. September 27. 2018
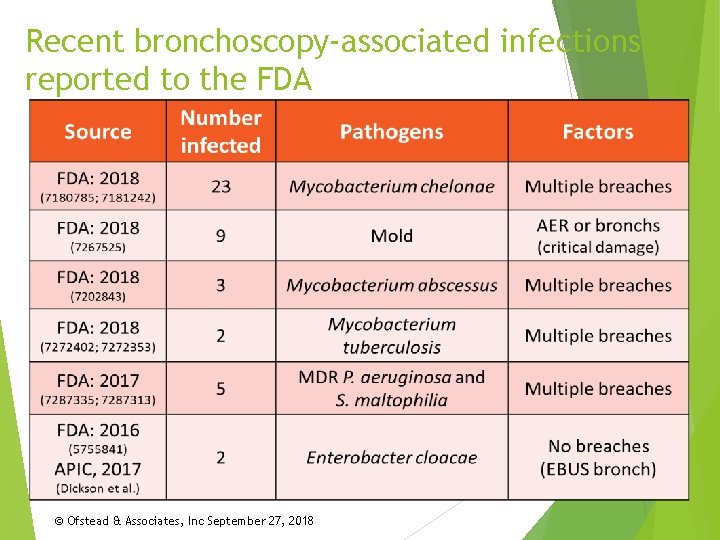
Recent bronchoscopy-associated infections reported to the FDA © Ofstead & Associates, Inc September 27, 2018
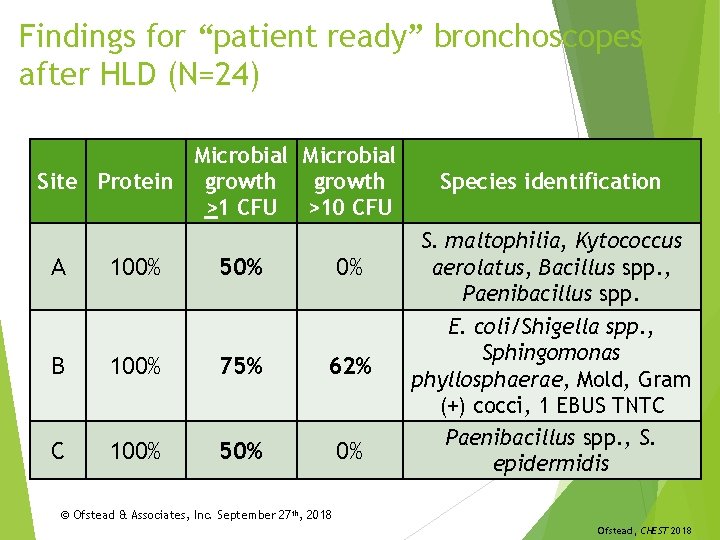
Findings for “patient ready” bronchoscopes after HLD (N=24) Microbial Site Protein growth >1 CFU >10 CFU A 100% 50% 0% B 100% 75% 62% C 100% 50% 0% Species identification S. maltophilia, Kytococcus aerolatus, Bacillus spp. , Paenibacillus spp. E. coli/Shigella spp. , Sphingomonas phyllosphaerae, Mold, Gram (+) cocci, 1 EBUS TNTC Paenibacillus spp. , S. epidermidis © Ofstead & Associates, Inc. September 27 th, 2018 Ofstead, CHEST 2018

Simethicone and Non-Water Soluble Additives Simethicone usage in GI scopes creates an unknown risk Silicone and sugar = ideal growth medium for bacteria Challenges in removing from equipment SGNA states to minimize use of simethicone pending further studies 6/5/2021 38
Ultrasound Probes Transvaginal/Transrectal probes and High-Risk HPV Non-oxidative HLD agents not reliably killing these organisms Trophon has been game changer TEE probes Unknown risk with HPV due to increased incidence of esophageal cancers from HPV Most cannot be disinfected with oxidative HLD agents 6/5/2021 39

Failure to Follow Disinfection Principles Watch for these events in your facility Human errors: inadequate pre-cleaning, inadequate exposure times, documentation failures, poor IC practices (cross contamination, PPE use), improper use of detergents/disinfectants Equipment: no preventive maintenance, not tested/cleaned, lack of knowledge on use 6/5/20 21 40

What can IPs do to help minimize risk? Review IFUs and guidelines (e. g. , AAMI ST 91, AORN) Get training as needed to provide leadership Assess technician training and competency testing Arrange for an expert to audit reprocessing practices Implement methods for monitoring quality and outcomes Use available resources, such as: Guideline crosswalk in “A glimpse of the true cost of reprocessing endoscopes”: https: //ofsteadinsights. com/? page_id=18 IAHCSMM’s Endoscope Reprocessing Manual (new textbook for training techs): https: //www. iahcsmm. org/publications CDC/HICPAC recommendations and toolkit: https: //www. cdc. gov/hicpac/recommendations/flexibleendoscope-reprocessing. html © Ofstead & Associates, Inc September 27, 2018

What else can IPs do to reduce risk? Address issues that may be contributing to risk: Cultural issues that lead to cutting corners: Unaddressed ergonomic and occupational health issues Pressure to go faster (efficiency valued over quality) Bullying of reprocessing personnel Inadequate bedside pre-cleaning and delayed reprocessing The co-mingling of GI endoscope and bronchoscopes Water quality issues in the reprocessing suite The use of lubricants that cannot be removed The continued use of damaged scopes Move toward the use of sterilized endoscopes
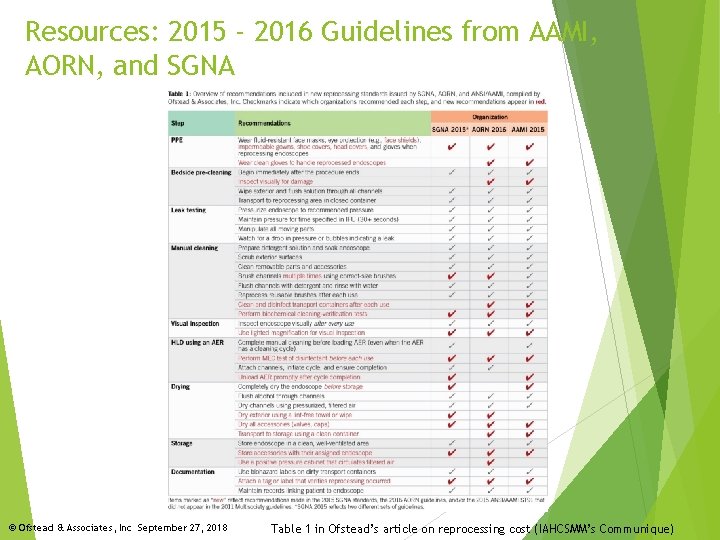
Resources: 2015 - 2016 Guidelines from AAMI, AORN, and SGNA © Ofstead & Associates, Inc September 27, 2018 Table 1 in Ofstead’s article on reprocessing cost (IAHCSMM’s Communique)

Resources: IAHCSMM’s textbook on reprocessing Includes: Regulations, Standards and Resources Point-of-Use Cleaning, Transport and Leak Testing Cleaning Processes for Flexible Endoscopes Endoscope Inspection and Preparation High-Level Disinfection and Sterilization processes for Flexible Endoscopes Endoscope Handling, Storage and Transport https: //www. iahcsmm. org/publications/endoscope-reprocessing. html © Ofstead & Associates, Inc September 27, 2018

6 pages of easy-to-understand recommendations https: //www. cdc. gov/hicpac/recommendations/flexible-endoscope-reprocessing. html © Ofstead & Associates, Inc September 27, 2018

References Association for Professionals in Infection Control. (2009). APIC Text of Infection Control and Epidemiology, 3 rd Edition. Retrieved from: http: //www. apic. org/Content/Navigation. Menu/Links/Publications/APICT ext/APIC_Text_Table_of_Contents. pdf Association of Peri. Operative Registered Nurses. (2013). Perioperative Standards and Recommended Practices. Association for the Advancement of Medical Instrumentation. (2015). ANSI/AAMI ST 91 Flexible and Semi-Rigid Endoscope Reprocessing in Health Care Facilities. Bennett, G. & Kassai, M. (2011). Infection Prevention Manual for Ambulatory Surgery Centers. ICP Associates Update: Rutala W. A. , Weber D. J. , and the Hospital Infection Control Practices Advisory Committee. (2008). Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. Centers for Disease Control and Prevention 6/5/20 21 46

References Update: Rutala W. A. , and Weber D. J. , “Disinfection and Sterilization in Healthcare Facilities: What Clinicians Need to Know, ” Clinical Infectious Diseases (2004); 39: 702 -9. CDC Health Alert Network, CDC Health Advisory, September 11, 2015. CDC Health Advisory US Food and Drug Administration. Liquid Chemical Sterilization American Society for Gastrointestinal Endoscopy. Multisociety guideline on reprocessing flexible gastrointestinal endoscopes: 2011. Volume 73, No. 6 : 2011. Retrieved from: http: //www. asge. org/uploaded. Files/Public_EBlast_PDFs/Reprocessing. Endoscopes. pdf 6/5/20 21 47
- Slides: 47