Higher Physics Unit 2 Particles and Waves Photoelectric














- Slides: 23

Higher Physics

Unit 2 Particles and Waves

Photoelectric Emission

Introduction (don’t copy) In some experiments (e. g. interference), light and the other forms of electromagnetic radiation behave like continuous waves of energy, but in the photoelectric effect they behave like individual “lumps” or “packets” of energy called photons. Electrons are knocked off a metal surface by photons. The electrons can form a photoelectric current.

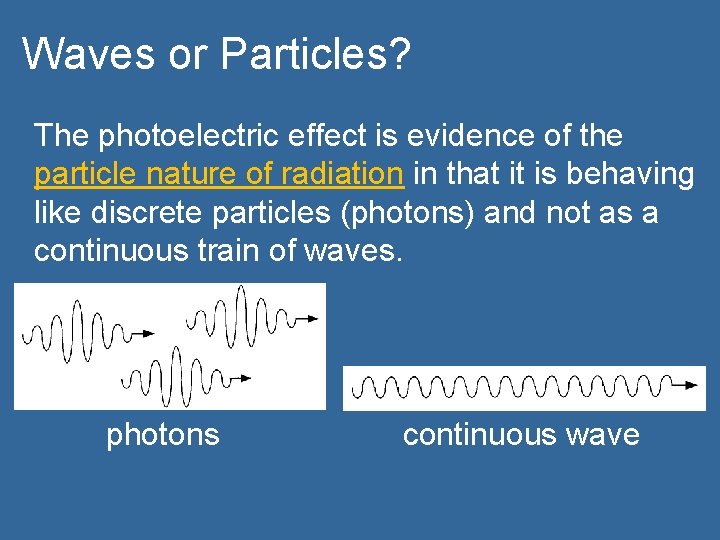
Waves or Particles? The photoelectric effect is evidence of the particle nature of radiation in that it is behaving like discrete particles (photons) and not as a continuous train of waves. photons continuous wave

https: //www. youtube. com/watch? v=h 1 tfl. E-L 2 Dc

Photons A beam of radiation can be regarded as a stream of individual energy bundles called photons. Each photon has an energy E = hf h = Planck’s constant (6. 63 x 10 -34 Js) f = frequency of the radiation

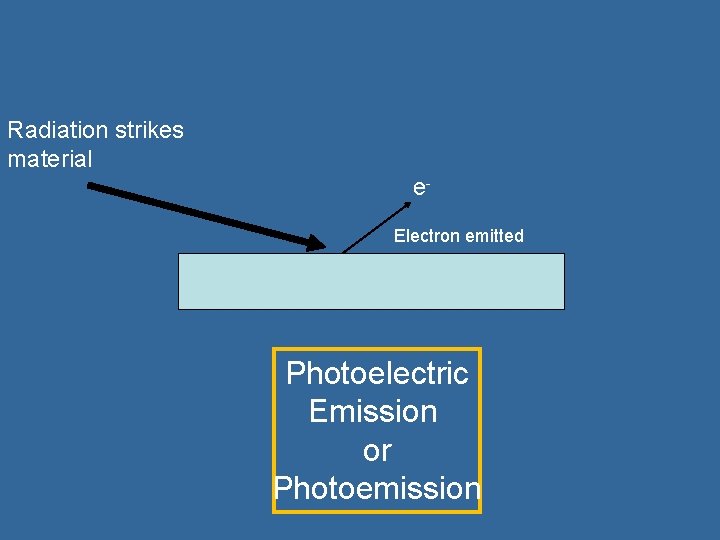
Photoelectric Emission Sometimes when electromagnetic radiation above a certain frequency strikes a surface, electrons are emitted. The electrons can form a photoelectric current. This can be used to detect radiation, and is the basis on which photodiodes, solar cells and LDRs operate.

Radiation strikes material e. Electron emitted Photoelectric Emission or Photoemission

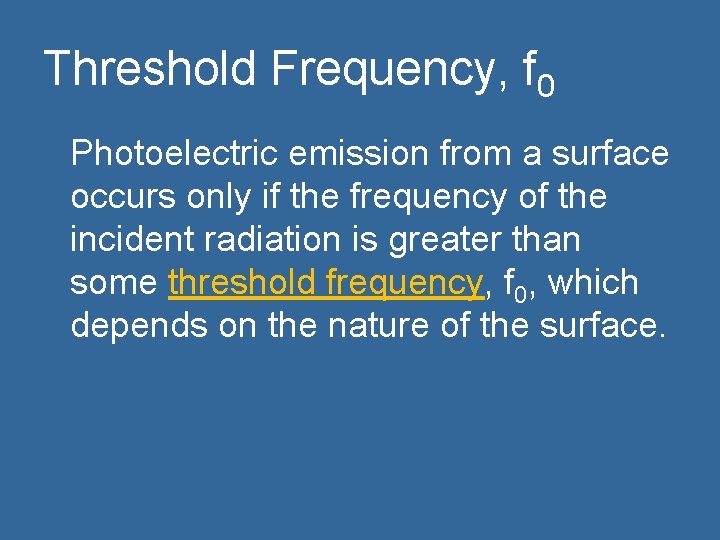
Threshold Frequency, f 0 Photoelectric emission from a surface occurs only if the frequency of the incident radiation is greater than some threshold frequency, f 0, which depends on the nature of the surface.

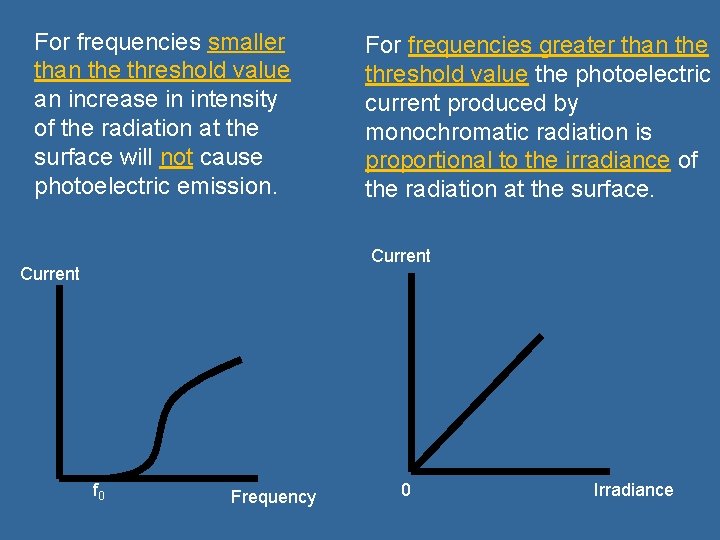
For frequencies smaller than the threshold value an increase in intensity of the radiation at the surface will not cause photoelectric emission. For frequencies greater than the threshold value the photoelectric current produced by monochromatic radiation is proportional to the irradiance of the radiation at the surface. Current f 0 Frequency 0 Irradiance

If N photons per second are incident per unit area on a surface the irradiance at the surface is: I = Nhf

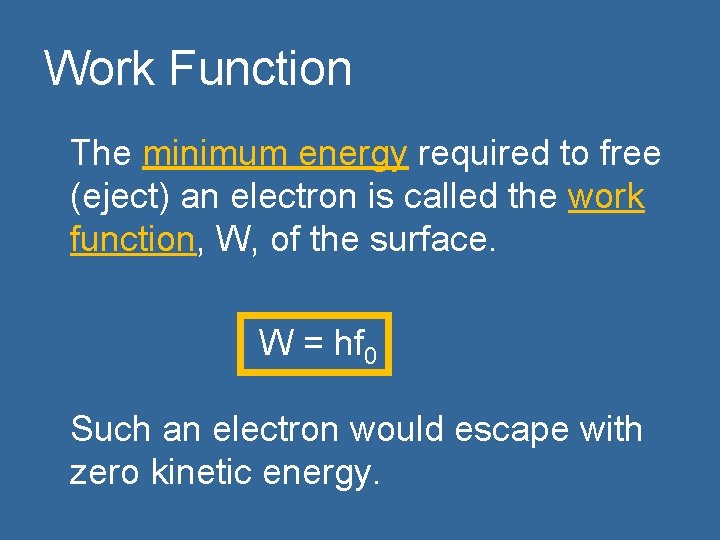
Work Function The minimum energy required to free (eject) an electron is called the work function, W, of the surface. W = hf 0 Such an electron would escape with zero kinetic energy.

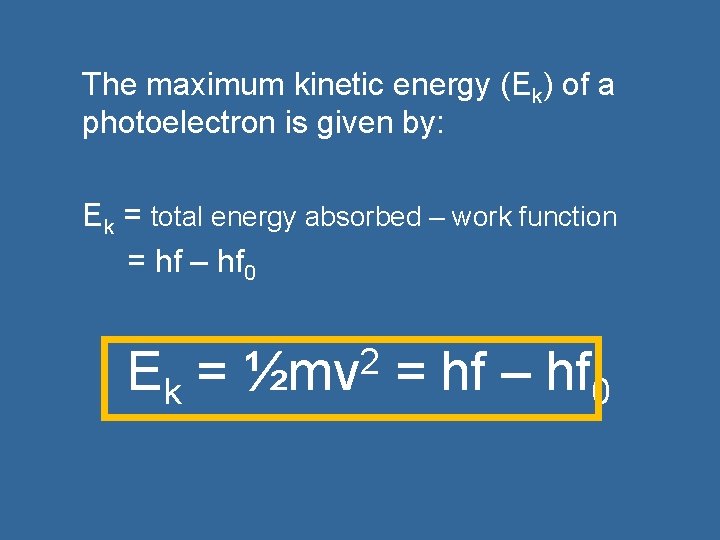
The maximum kinetic energy (Ek) of a photoelectron is given by: Ek = total energy absorbed – work function = hf – hf 0 Ek = 2 ½mv = hf – hf 0

Example A metal has a work function of 1. 8 x 10 -20 J. a) Find the kinetic energy of an ejected electron if a photon striking the metal has a frequency of 3. 1 x 1013 Hz. b) What will be the maximum possible velocity of the ejected electrons?

https: //www. youtube. com/watch? v=v-1 zjd. UTu 0 o

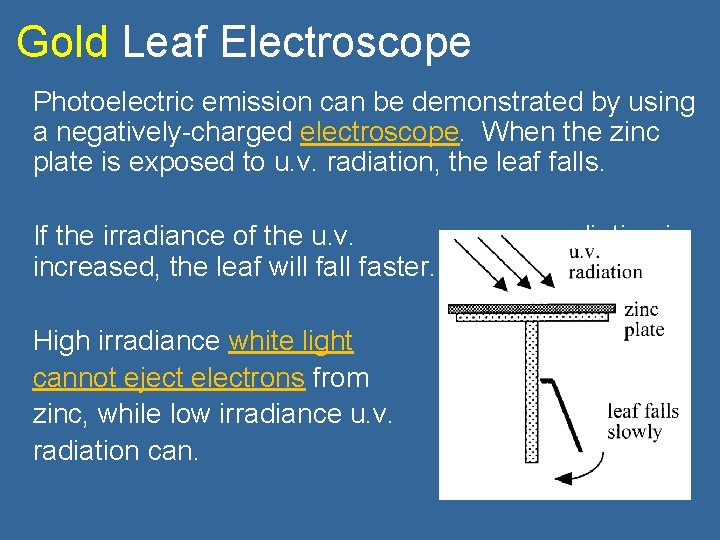
Gold Leaf Electroscope Photoelectric emission can be demonstrated by using a negatively-charged electroscope. When the zinc plate is exposed to u. v. radiation, the leaf falls. If the irradiance of the u. v. increased, the leaf will faster. High irradiance white light cannot eject electrons from zinc, while low irradiance u. v. radiation can. radiation is

2010 Q 14

2006 Q 17

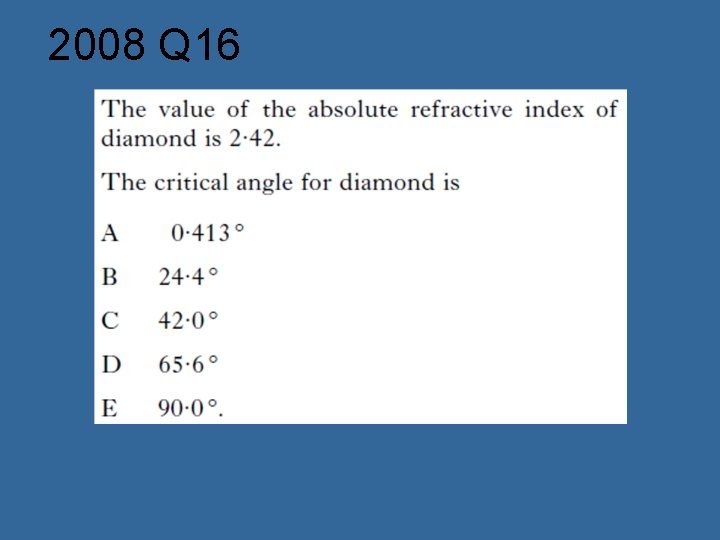
2008 Q 16

2004 Q 15

2009 Q 15

2001 Q 19