High Throughput Synthesis Within Flow Reactors Paul Watts



- Slides: 24

High Throughput Synthesis Within Flow Reactors Paul Watts CPAC, Rome, March 19 th 2007

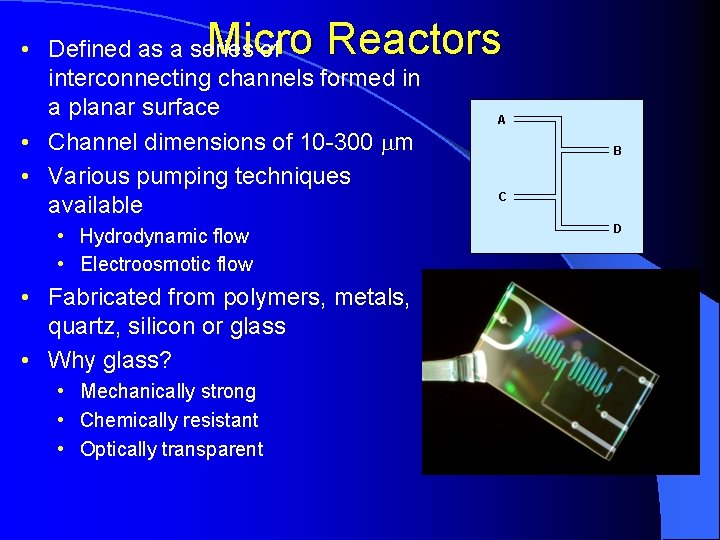
Micro Reactors • Defined as a series of interconnecting channels formed in a planar surface • Channel dimensions of 10 -300 mm • Various pumping techniques available • Hydrodynamic flow • Electroosmotic flow • Fabricated from polymers, metals, quartz, silicon or glass • Why glass? • Mechanically strong • Chemically resistant • Optically transparent A B C D

PET Radiosynthesis • Positron emission tomography (PET) is a radiotracer imaging technique used to provide quantitative information on physiological and biochemical phenomena in vivo • Applications in clinical research and drug discovery • Two of the most desirable radioisotopes are: • • 11 C (t 1/2 20. 4 minutes) 18 F (t 1/2 109. 7 minutes) • Syntheses must be conducted within 2 -3 half-lives • Aims of miniaturisation: • Produce the desired quantity of radiotracer (< 1 mg) at point of use • Reduced reaction times will produce the product with enhanced specific activity • The PET ligand will have greater sensitivity in vivo Collaboration with NIH, Washington DC

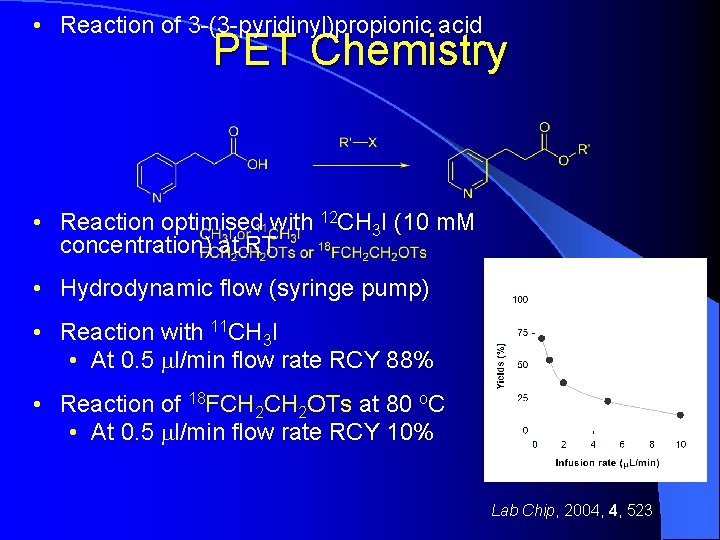
• Reaction of 3 -(3 -pyridinyl)propionic acid PET Chemistry • Reaction optimised with 12 CH 3 I (10 m. M concentration) at RT • Hydrodynamic flow (syringe pump) • Reaction with 11 CH 3 I • At 0. 5 ml/min flow rate RCY 88% • Reaction of 18 FCH 2 OTs at 80 o. C • At 0. 5 ml/min flow rate RCY 10% Lab Chip, 2004, 4, 523

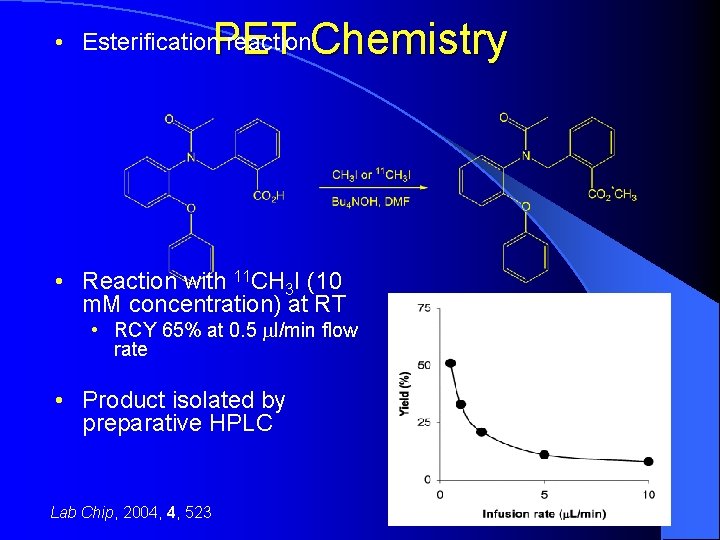
PET Chemistry • Esterification reaction • Reaction with 11 CH 3 I (10 m. M concentration) at RT • RCY 65% at 0. 5 ml/min flow rate • Product isolated by preparative HPLC Lab Chip, 2004, 4, 523

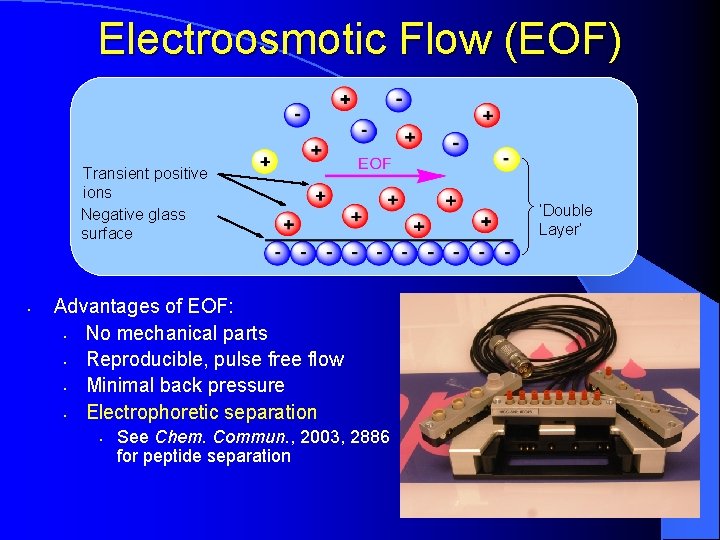
Electroosmotic Flow (EOF) Transient positive ions Negative glass surface • Advantages of EOF: • No mechanical parts • Reproducible, pulse free flow • Minimal back pressure • Electrophoretic separation • See Chem. Commun. , 2003, 2886 for peptide separation ‘Double Layer’

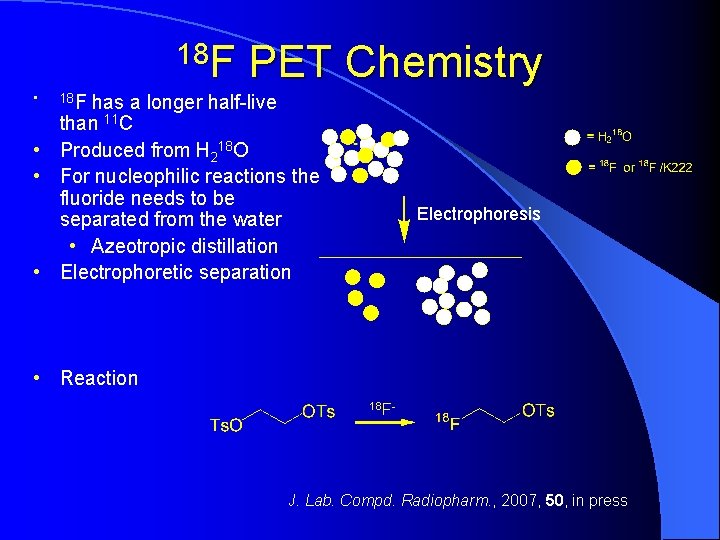
18 F • PET Chemistry 18 F has a longer half-live than 11 C • Produced from H 218 O • For nucleophilic reactions the fluoride needs to be separated from the water • Azeotropic distillation • Electrophoretic separation Electrophoresis • Reaction 18 F- J. Lab. Compd. Radiopharm. , 2007, 50, in press

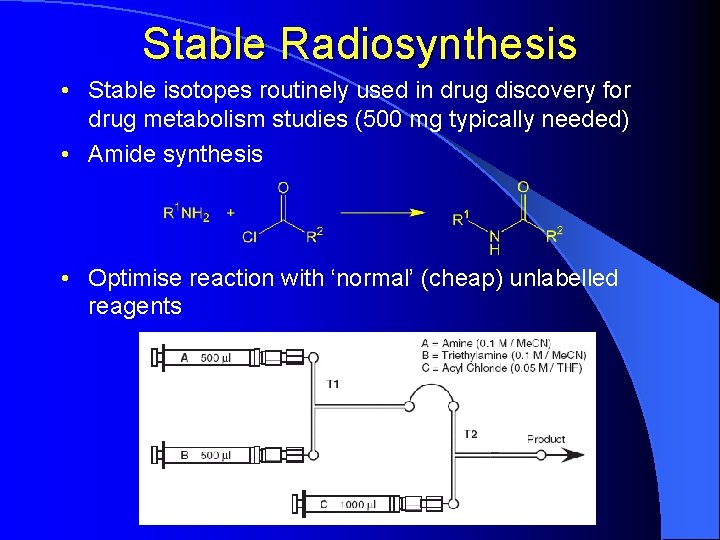
Stable Radiosynthesis • Stable isotopes routinely used in drug discovery for drug metabolism studies (500 mg typically needed) • Amide synthesis • Optimise reaction with ‘normal’ (cheap) unlabelled reagents

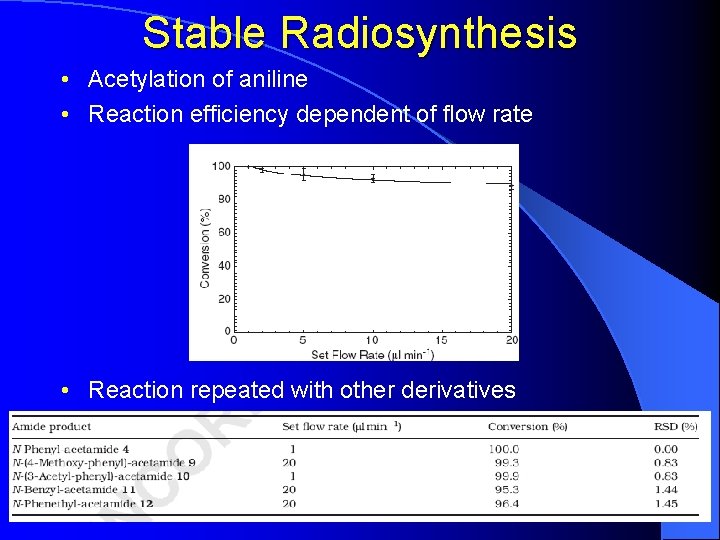
Stable Radiosynthesis • Acetylation of aniline • Reaction efficiency dependent of flow rate • Reaction repeated with other derivatives

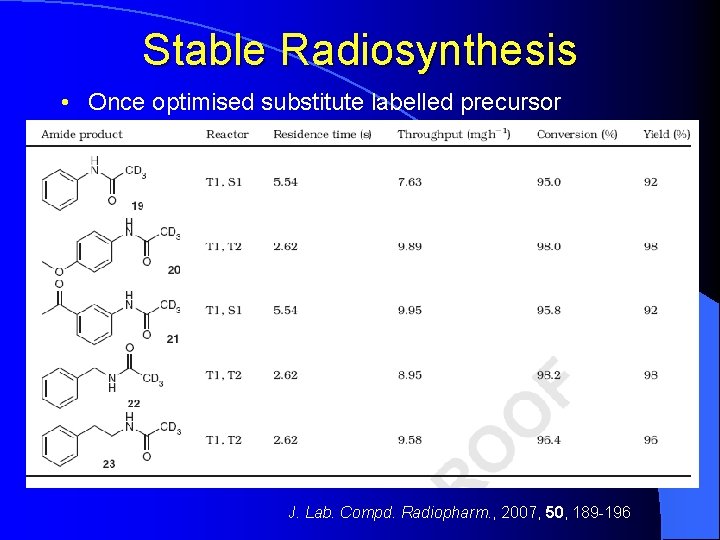
Stable Radiosynthesis • Once optimised substitute labelled precursor J. Lab. Compd. Radiopharm. , 2007, 50, 189 -196

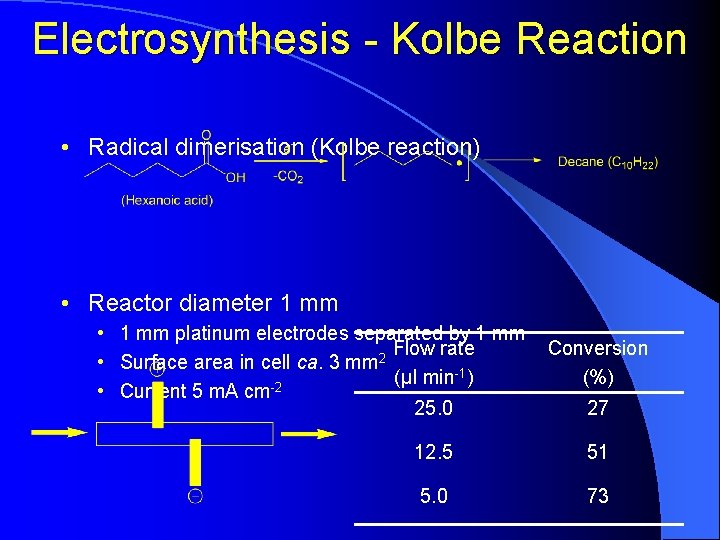
Electrosynthesis - Kolbe Reaction • Radical dimerisation (Kolbe reaction) • Reactor diameter 1 mm • 1 mm platinum electrodes separated by 1 mm Flow rate 2 • Surface area in cell ca. 3 mm (μl min-1) -2 • Current 5 m. A cm 25. 0 Conversion (%) 27 12. 5 51 5. 0 73

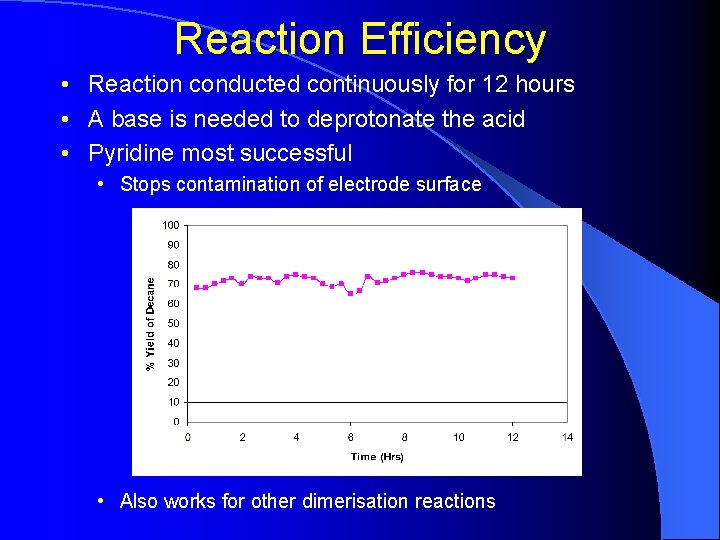
Reaction Efficiency • Reaction conducted continuously for 12 hours • A base is needed to deprotonate the acid • Pyridine most successful • Stops contamination of electrode surface • Also works for other dimerisation reactions

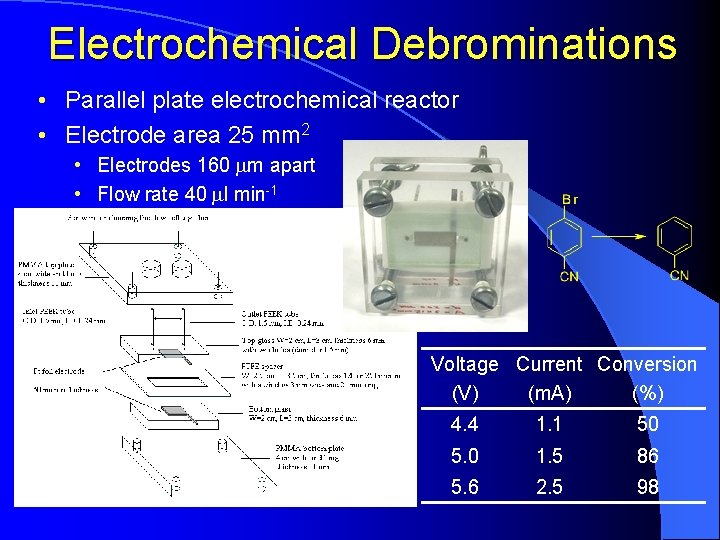
Electrochemical Debrominations • Parallel plate electrochemical reactor • Electrode area 25 mm 2 • Electrodes 160 mm apart • Flow rate 40 ml min-1 Voltage Current Conversion (V) (m. A) (%) 4. 4 1. 1 50 5. 0 1. 5 86 5. 6 2. 5 98

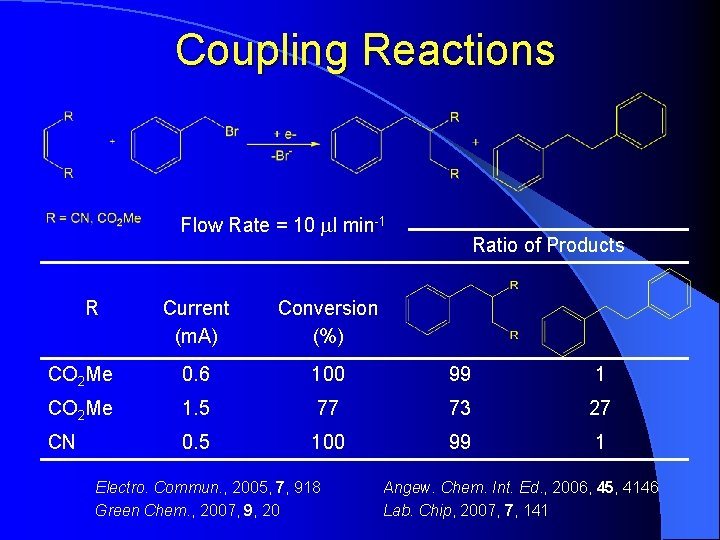
Coupling Reactions Flow Rate = 10 ml min-1 R Ratio of Products Current (m. A) Conversion (%) CO 2 Me 0. 6 100 99 1 CO 2 Me 1. 5 77 73 27 CN 0. 5 100 99 1 Electro. Commun. , 2005, 7, 918 Green Chem. , 2007, 9, 20 Angew. Chem. Int. Ed. , 2006, 45, 4146 Lab. Chip, 2007, 7, 141

Fine Chemical Synthesis • New methodology for fine chemical synthesis • Enhanced yields of more pure products etc • But always require purification • Generally batch work up required

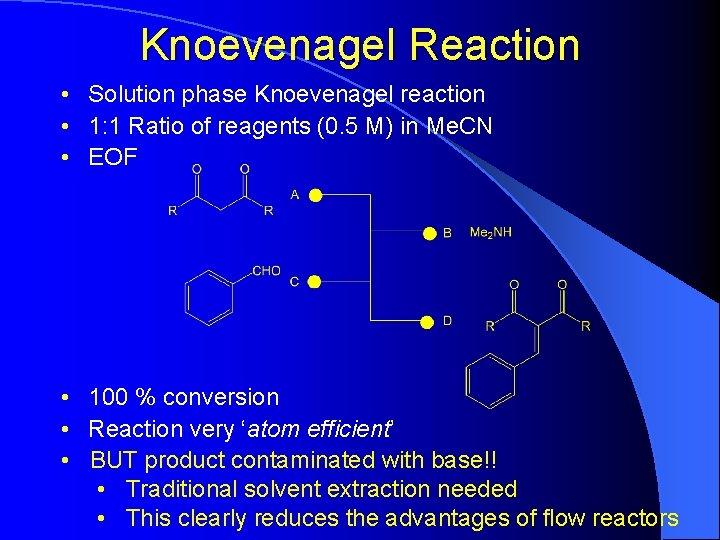
Knoevenagel Reaction • Solution phase Knoevenagel reaction • 1: 1 Ratio of reagents (0. 5 M) in Me. CN • EOF • 100 % conversion • Reaction very ‘atom efficient’ • BUT product contaminated with base!! • Traditional solvent extraction needed • This clearly reduces the advantages of flow reactors

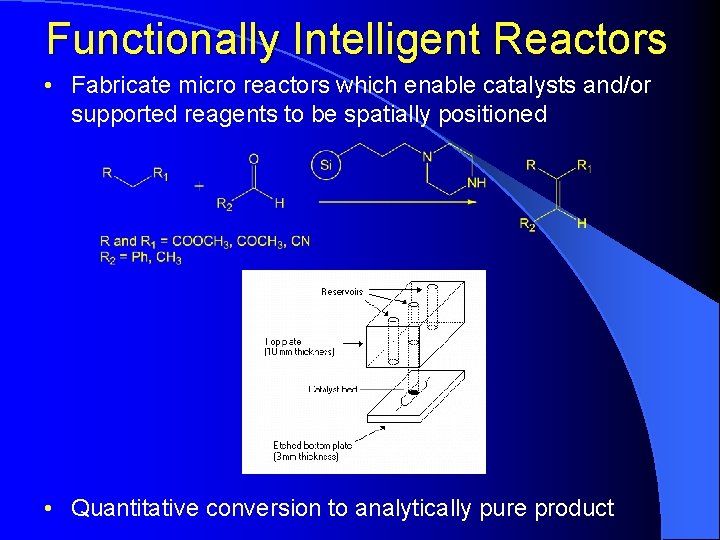
Functionally Intelligent Reactors • Fabricate micro reactors which enable catalysts and/or supported reagents to be spatially positioned • Quantitative conversion to analytically pure product

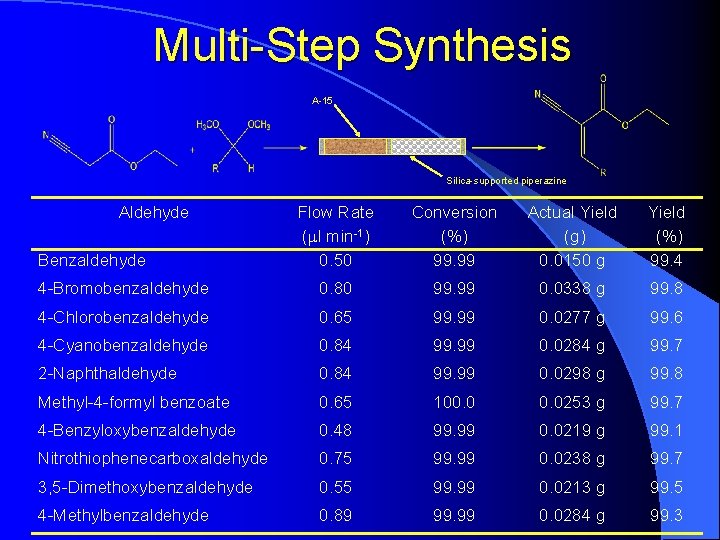
Multi-Step Synthesis A-15 Silica-supported piperazine Aldehyde Flow Rate (ml min-1) 0. 50 Conversion (%) 99. 99 Actual Yield (g) 0. 0150 g Yield (%) 99. 4 4 -Bromobenzaldehyde 0. 80 99. 99 0. 0338 g 99. 8 4 -Chlorobenzaldehyde 0. 65 99. 99 0. 0277 g 99. 6 4 -Cyanobenzaldehyde 0. 84 99. 99 0. 0284 g 99. 7 2 -Naphthaldehyde 0. 84 99. 99 0. 0298 g 99. 8 Methyl-4 -formyl benzoate 0. 65 100. 0253 g 99. 7 4 -Benzyloxybenzaldehyde 0. 48 99. 99 0. 0219 g 99. 1 Nitrothiophenecarboxaldehyde 0. 75 99. 99 0. 0238 g 99. 7 3, 5 -Dimethoxybenzaldehyde 0. 55 99. 99 0. 0213 g 99. 5 4 -Methylbenzaldehyde 0. 89 99. 99 0. 0284 g 99. 3 Benzaldehyde

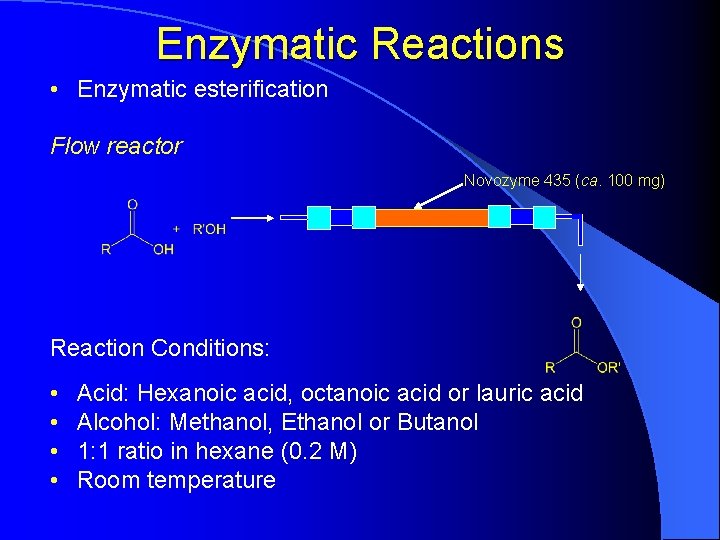
Enzymatic Reactions • Enzymatic esterification Flow reactor Novozyme 435 (ca. 100 mg) Reaction Conditions: • • Acid: Hexanoic acid, octanoic acid or lauric acid Alcohol: Methanol, Ethanol or Butanol 1: 1 ratio in hexane (0. 2 M) Room temperature

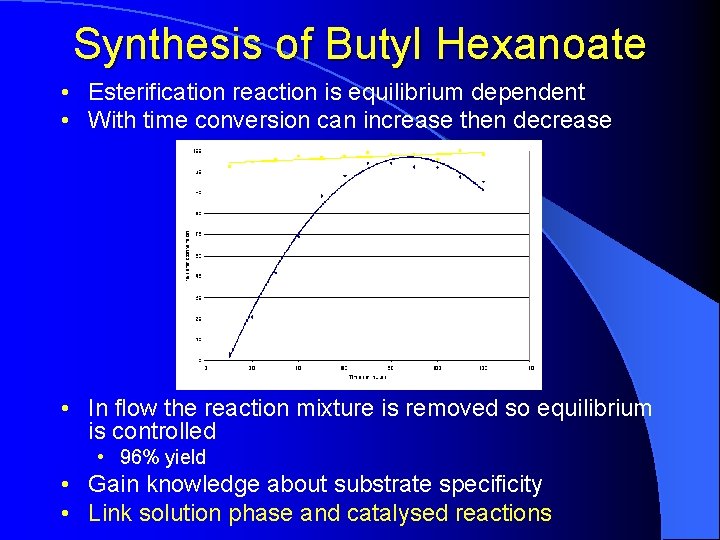
Synthesis of Butyl Hexanoate • Esterification reaction is equilibrium dependent • With time conversion can increase then decrease • In flow the reaction mixture is removed so equilibrium is controlled • 96% yield • Gain knowledge about substrate specificity • Link solution phase and catalysed reactions

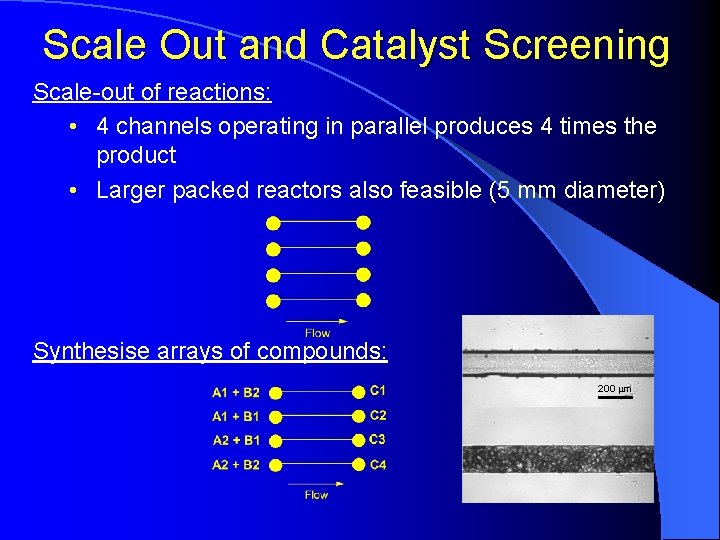
Scale Out and Catalyst Screening Scale-out of reactions: • 4 channels operating in parallel produces 4 times the product • Larger packed reactors also feasible (5 mm diameter) Synthesise arrays of compounds: 200 mm

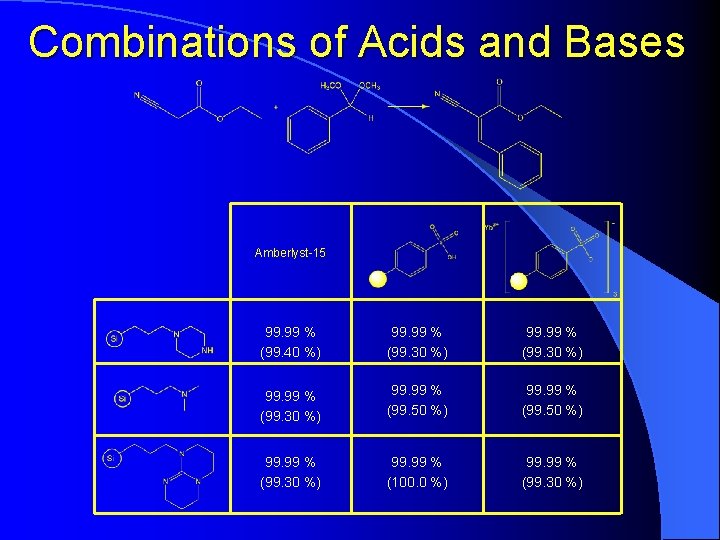
Combinations of Acids and Bases Amberlyst-15 99. 99 % (99. 40 %) 99. 99 % (99. 30 %) 99. 99 % (99. 50 %) 99. 99 % (99. 30 %) 99. 99 % (100. 0 %) 99. 99 % (99. 30 %)

Conclusions • Micro reactors allow the rapid optimisation of reactions • High-throughput combinatorial synthesis • Immobilised reagents (catalysts and enzymes) allow the synthesis of analytically pure compounds • Micro reactors are suitable for a wide range of reactions • Electrochemical synthesis • Catalysed reactions • Enzyme screening • Micro reactors generate products in: • Higher purity • Higher conversion • Higher selectivity • In situ formation of reagents P. D. I. Fletcher et al. , Tetrahedron, 2002, 58, 4735 K. Jahnisch et al. , Angew. Chem. Int. Ed. , 2004, 43, 406 H. Pennemann et al. , OPRD, 2004, 8, 422 P. Watts et al. , Chem. Soc. Rev. , 2005, 34, 235

Research Workers and Collaborators • • • Dr. Charlotte Wiles Dr. Nikzad Nikbin Dr. Ping He Dr. Victoria Ryabova Dr. Vinod George Dr. Leanne Marle Dr. Joe Dragavon Lioni. X Astra Zeneca Novartis • • Mairead Kelly Gareth Wild Tamsila Nayyar Julian Hooper Linda Woodcock Haider Al-Lawati Ben Wahab • EPSRC • Sanofi-Aventis • EU FP 6