High temperature steam electrolysis HTSE for hydrogen production


- Slides: 16

High temperature steam electrolysis (HTSE) for hydrogen production: from material developement to stack operation Julie Mougin, G. Gousseau, B. Morel, F. Lefebvre. Joud, F. Le Naour, F. Chauveau, J. C. Grenier CEA-Grenoble, France ICMCB-Bordeaux, France Fourth NEA Information Exchange Meeting on Nuclear Production of Hydrogen Chicago, Oakbrook, 13 – 16 April 2009 NEA Chicago April 14 th 2009 1

HTSE: from material development to stack operation Outline 8 Introduction 8 Experimental 4 Stack/SRU design 4 New cell electrode material 8 Results 4 Stack performances and short-term durability 4 SRU performances 4 New cell electrode performance 8 Conclusions NEA Chicago April 14 th 2009 2

Introduction 8 High temperature steam electrolysis (HTSE) = one of the most promising “clean” processes for massive production of hydrogen 8 Nuclear energy considered to provide electricity and heat to split water molecule into H 2 and O 2 8 Necessity of highly efficient systems in order to avoid too many dedicated nuclear power plants each component has to be optimized, from the balance of plant to the stack and to the solid oxide electrolyzer cell (SOEC) 8 CEA (French Atomic Energy Commission) is carrying out researches in this field: Stack design Several designs specific to HTSE One low-weight stack Instrumented SRU (single repeat unit) To identify and understand specificities of stack environment To investigate new solutions in stack environment Materials and components To increase hydrogen production and durability Cell materials, coatings and sealings NEA Chicago April 14 th 2009 3

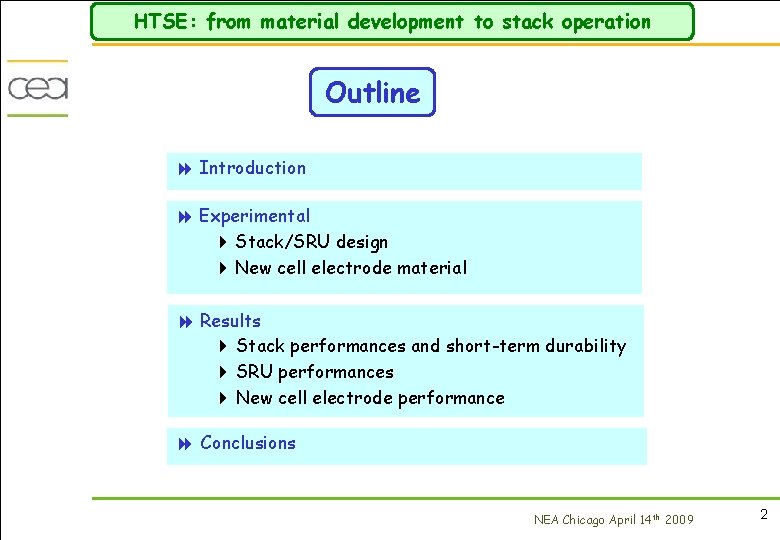
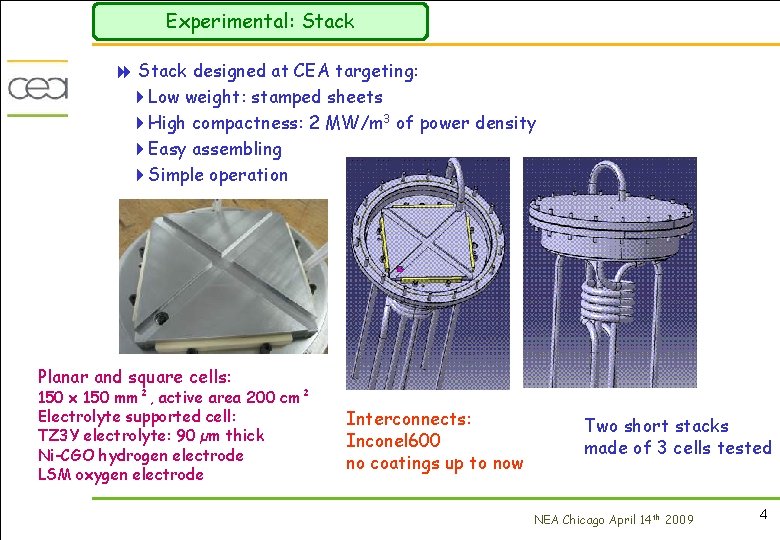
Experimental: Stack 8 Stack designed at CEA targeting: 4 Low weight: stamped sheets 4 High compactness: 2 MW/m 3 of power density 4 Easy assembling 4 Simple operation Planar and square cells: 150 x 150 mm², active area 200 cm² Electrolyte supported cell: TZ 3 Y electrolyte: 90 µm thick Ni-CGO hydrogen electrode LSM oxygen electrode Interconnects: Inconel 600 no coatings up to now Two short stacks made of 3 cells tested NEA Chicago April 14 th 2009 4

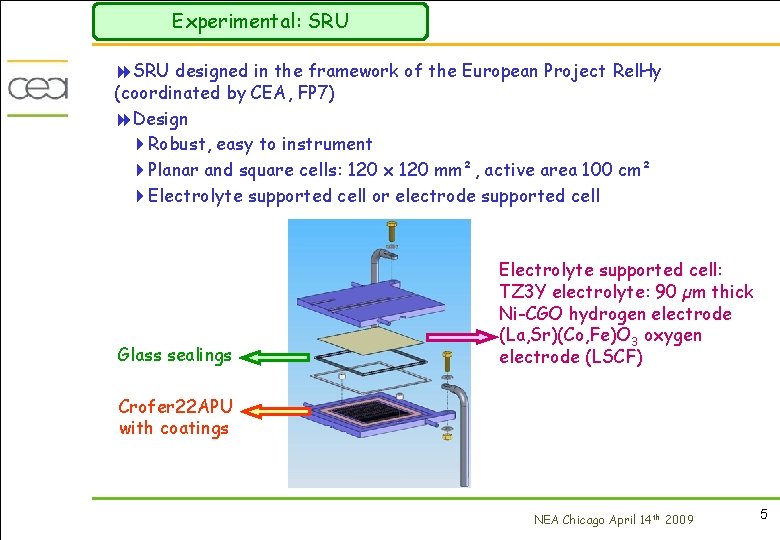
Experimental: SRU 8 SRU designed in the framework of the European Project Rel. Hy (coordinated by CEA, FP 7) 8 Design 4 Robust, easy to instrument 4 Planar and square cells: 120 x 120 mm², active area 100 cm² 4 Electrolyte supported cell or electrode supported cell Glass sealings Electrolyte supported cell: TZ 3 Y electrolyte: 90 µm thick Ni-CGO hydrogen electrode (La, Sr)(Co, Fe)O 3 oxygen electrode (LSCF) Crofer 22 APU with coatings NEA Chicago April 14 th 2009 5

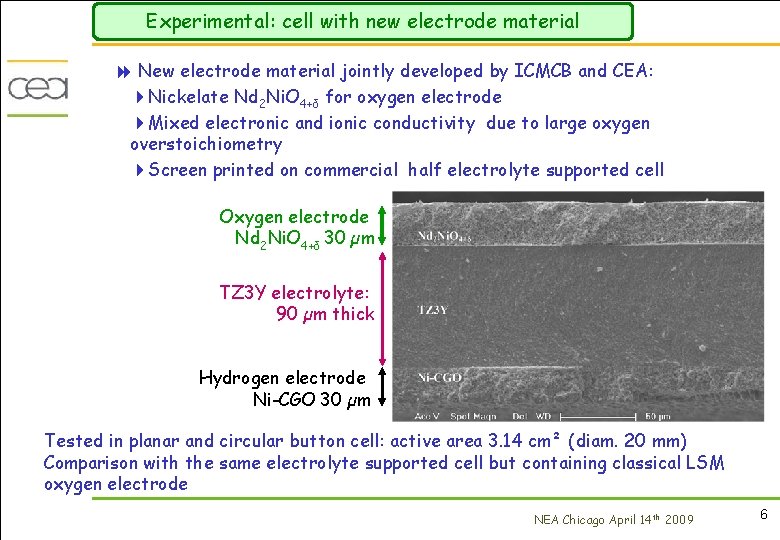
Experimental: cell with new electrode material 8 New electrode material jointly developed by ICMCB and CEA: 4 Nickelate Nd 2 Ni. O 4+δ for oxygen electrode 4 Mixed electronic and ionic conductivity due to large oxygen overstoichiometry 4 Screen printed on commercial half electrolyte supported cell Oxygen electrode Nd 2 Ni. O 4+δ 30 µm TZ 3 Y electrolyte: 90 µm thick Hydrogen electrode Ni-CGO 30 µm Tested in planar and circular button cell: active area 3. 14 cm² (diam. 20 mm) Comparison with the same electrolyte supported cell but containing classical LSM oxygen electrode NEA Chicago April 14 th 2009 6

Experimental: testing procedure 8 Stack: T = 820°C Pure water vapor at the hydrogen electrode 8 SRU: T = 800°C Several ratio of H 2 O/H 2 to the hydrogen electrode 90% H 2 O / 10% H 2 70% H 2 O / (10% H 2 / 20% N 2) 50% H 2 O / (10% H 2 / 40% N 2) 8 Cell: T = 750 -800 -850°C Gas to the hydrogen electrode: 35% H 2 O / 37% H 2 / 28% Ar 8 Stack/SRU/cell Performance: i-V curve ASR measured at 1. 3 V 8 Stack durability: Under galvanostatic control to achieve ~ 1. 3 V per cell NEA Chicago April 14 th 2009 7

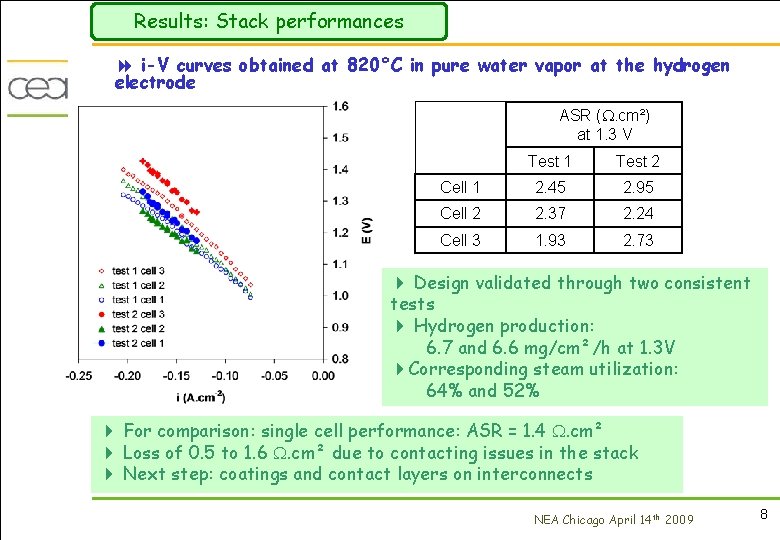
Results: Stack performances 8 i-V curves obtained at 820°C in pure water vapor at the hydrogen electrode ASR (. cm²) at 1. 3 V Test 1 Test 2 Cell 1 2. 45 2. 95 Cell 2 2. 37 2. 24 Cell 3 1. 93 2. 73 4 Design validated through two consistent tests 4 Hydrogen production: 6. 7 and 6. 6 mg/cm²/h at 1. 3 V 4 Corresponding steam utilization: 64% and 52% 4 For comparison: single cell performance: ASR = 1. 4 . cm² 4 Loss of 0. 5 to 1. 6 . cm² due to contacting issues in the stack 4 Next step: coatings and contact layers on interconnects NEA Chicago April 14 th 2009 8

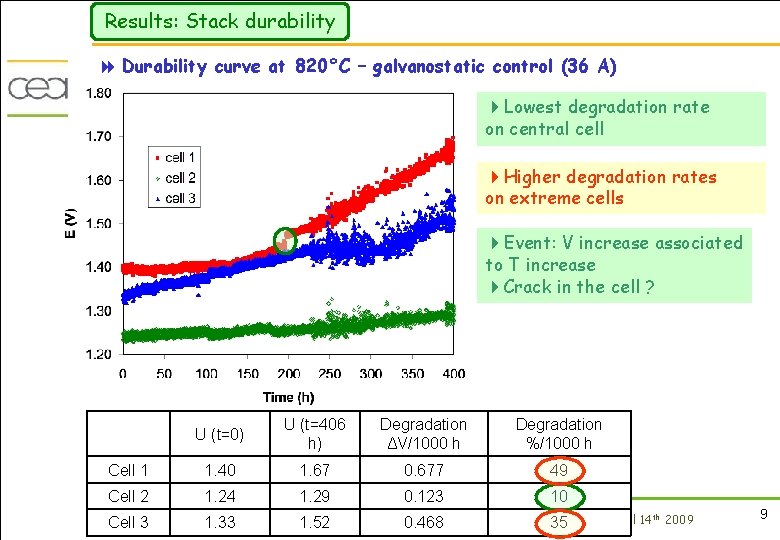
Results: Stack durability 8 Durability curve at 820°C – galvanostatic control (36 A) 4 Lowest degradation rate on central cell 4 Higher degradation rates on extreme cells 4 Event: V increase associated to T increase 4 Crack in the cell ? U (t=0) U (t=406 h) Degradation ΔV/1000 h Degradation %/1000 h Cell 1 1. 40 1. 67 0. 677 49 Cell 2 1. 24 1. 29 0. 123 10 Cell 3 1. 33 1. 52 0. 468 th NEA 35 Chicago April 14 2009 9

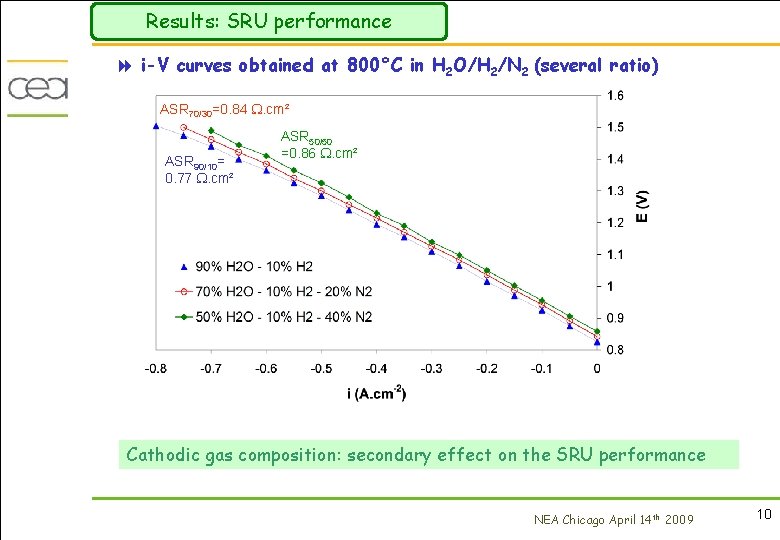
Results: SRU performance 8 i-V curves obtained at 800°C in H 2 O/H 2/N 2 (several ratio) ASR 70/30=0. 84 . cm² ASR 90/10= 0. 77 . cm² ASR 50/50 =0. 86 . cm² Cathodic gas composition: secondary effect on the SRU performance NEA Chicago April 14 th 2009 10

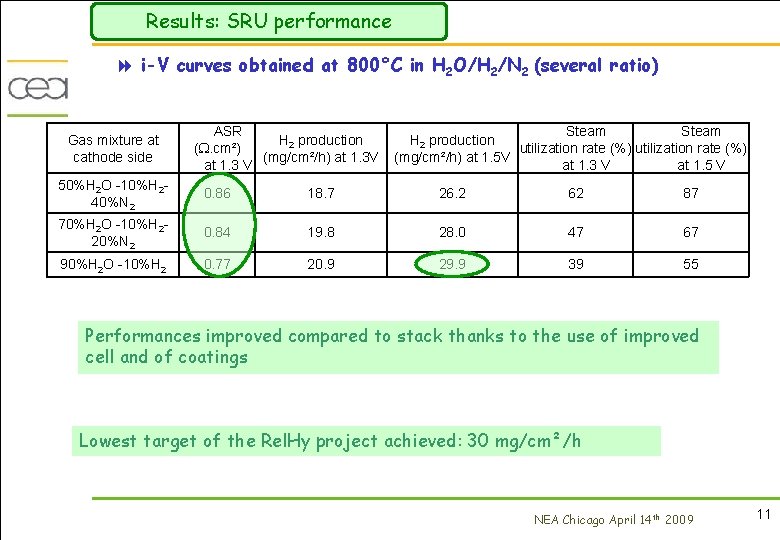
Results: SRU performance 8 i-V curves obtained at 800°C in H 2 O/H 2/N 2 (several ratio) Gas mixture at cathode side ASR H 2 production (. cm²) (mg/cm²/h) at 1. 3 V at 1. 3 V Steam H 2 production utilization rate (%) (mg/cm²/h) at 1. 5 V at 1. 3 V at 1. 5 V 50%H 2 O -10%H 240%N 2 0. 86 18. 7 26. 2 62 87 70%H 2 O -10%H 220%N 2 0. 84 19. 8 28. 0 47 67 90%H 2 O -10%H 2 0. 77 20. 9 29. 9 39 55 Performances improved compared to stack thanks to the use of improved cell and of coatings Lowest target of the Rel. Hy project achieved: 30 mg/cm²/h NEA Chicago April 14 th 2009 11

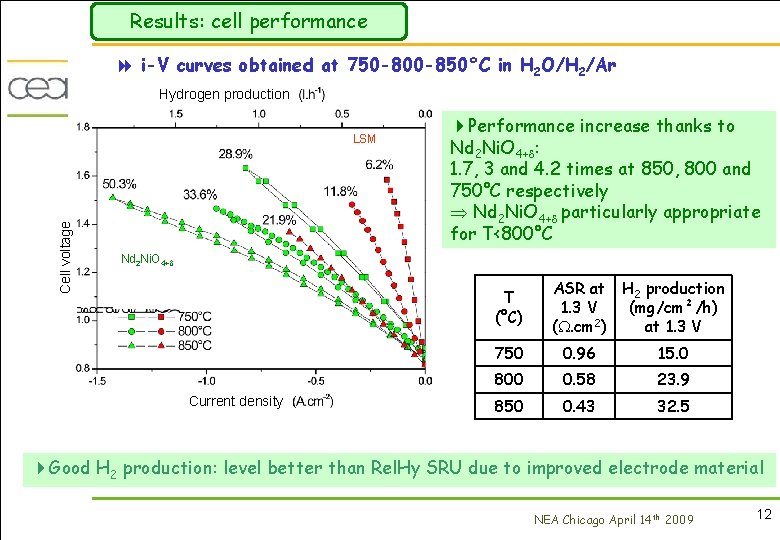
Results: cell performance 8 i-V curves obtained at 750 -800 -850°C in H 2 O/H 2/Ar Hydrogen production Cell voltage LSM 4 Performance increase thanks to Nd 2 Ni. O 4+ : 1. 7, 3 and 4. 2 times at 850, 800 and 750°C respectively Nd 2 Ni. O 4+ particularly appropriate for T<800°C Nd 2 Ni. O 4+ T (°C) Current density ASR at H 2 production 1. 3 V (mg/cm²/h) 2 (. cm ) at 1. 3 V 750 0. 96 15. 0 800 0. 58 23. 9 850 0. 43 32. 5 4 Good H 2 production: level better than Rel. Hy SRU due to improved electrode material NEA Chicago April 14 th 2009 12

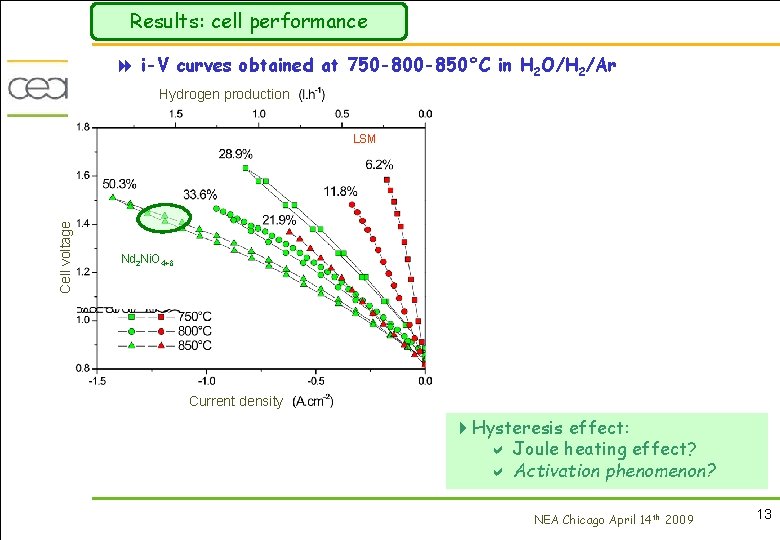
Results: cell performance 8 i-V curves obtained at 750 -800 -850°C in H 2 O/H 2/Ar Hydrogen production Cell voltage LSM Nd 2 Ni. O 4+ Current density 4 Hysteresis effect: a Joule heating effect? a Activation phenomenon? NEA Chicago April 14 th 2009 13

Conclusions 8 The stack design has been validated through two consistent tests of 3 cells stacks: ASR ~ 2 -3 . cm² at 820°C H 2 production ~ 7 mg/cm²/h at 1. 3 V Degradation rate evaluated for 400 hours: 10% /1000 h on central cell 35 and 49% /1000 h on extreme cells 8 SRU: ASR ~ 0. 8 -0. 9 . cm² at 800°C H 2 production ~ 20 mg/cm²/h at 1. 3 V 8 Cell with new oxygen electrode Nd 2 Ni. O 4+ : ASR ~ 0. 6 . cm² at 800°C H 2 production ~ 24 mg/cm²/h at 1. 3 V Performances 3 times higher than with regular LSM oxygen electrode Material particularly suitable at T<800°C NEA Chicago April 14 th 2009 14

Perspectives 8 Stack: Use of coatings and contact layers to improve performances and durability Test of 1 k. We stack 8 SRU: Durability test Further improvement of performances 8 Cell with new oxygen electrode Nd 2 Ni. O 4+ : Integration into large cells Test in stack/SRU environment Durability test NEA Chicago April 14 th 2009 15

Ackowledgments 8 Colleagues: From CEA: André Cahtroux, Patrick Mayoussier, Patrick Le Gallo, Pierre Baurens From ICMCB: Jean-Marc Bassat, Fabrice Mauvy From ECN: Jan-Pieter Ouweltjes 8 The French research Agency (ANR) and its Hydrogen and Fuel Cells program (Pan-H) for co-financing the SEMI-EHT project 8 The European Commission for co-financing the Rel. Hy project NEA Chicago April 14 th 2009 16