HIGH SPEED FLOW 1 st Semester 2007 Pawarej




- Slides: 20

HIGH SPEED FLOW 1 st Semester 2007 Pawarej CHOMDEJ fengpac@ku. ac. th 081 832 7854 05 -Jun-07 1

Course Outline 1. Introduction to compressible flows 2. Normal Shock Waves 3. Oblique Shock Waves 4. Prandtl - Mayer Flow 5. Application Involving Shocks and Expansion Fans 6. Flow with Friction 7. Flow with Heat Transfer ------------ Midterm Examination ------------8. Linearized Compressible Flow 9. Airfoils in Compressible Flows 05 -Jun-07 2

Course Outline 10. Wings and Wing-Fuselage Combinations in Compressible Flows 11. Method of Characteristics 12. Computational Gas Dynamics 13. Hypersonic Flows 05 -Jun-07 3

Course assessment • Attendance, Presentation, Quiz and Homework 40 points – Attendance – Presentation – Homework 10 points 20 points • Midterm examination • Final examination 05 -Jun-07 30 points 4

Introduction to compressible flows • Compressible flow – Review of thermodynamics – Total (Stagnation) conditions • Isentropic flow • Supersonic flow • Shock waves – Definition – Characteristics 05 -Jun-07 5

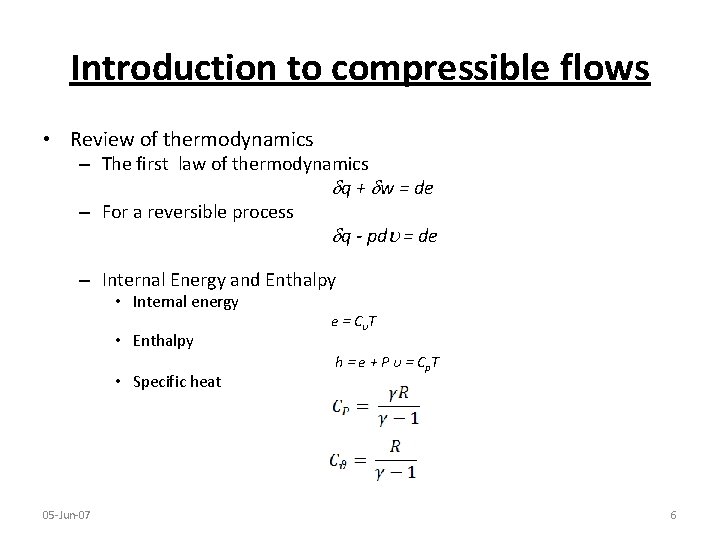
Introduction to compressible flows • Review of thermodynamics – The first law of thermodynamics q + w = de – For a reversible process q - pd = de – Internal Energy and Enthalpy • Internal energy • Enthalpy • Specific heat 05 -Jun-07 e = Cυ T h = e + P υ = Cp. T 6

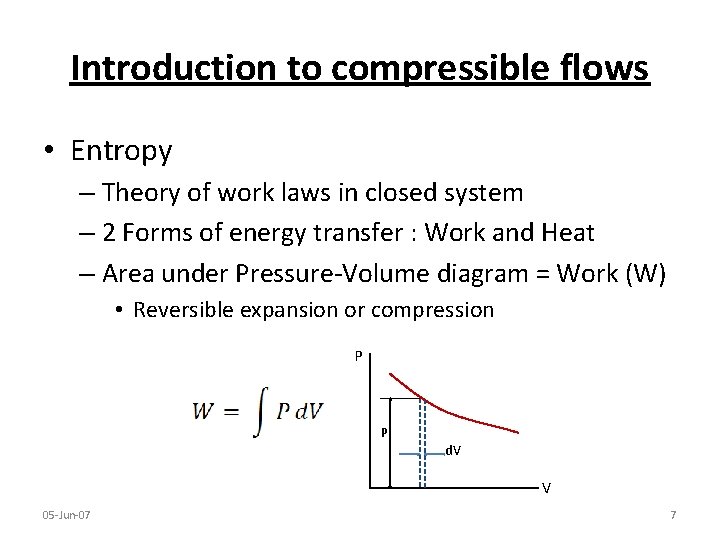
Introduction to compressible flows • Entropy – Theory of work laws in closed system – 2 Forms of energy transfer : Work and Heat – Area under Pressure-Volume diagram = Work (W) • Reversible expansion or compression P P d. V V 05 -Jun-07 7

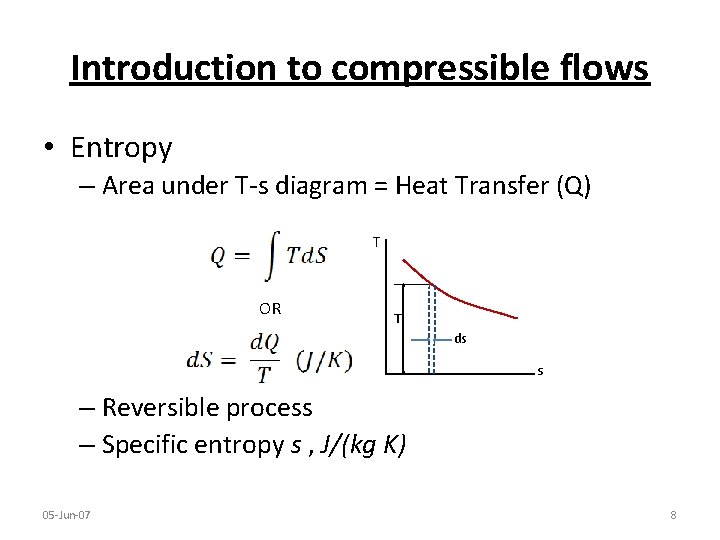
Introduction to compressible flows • Entropy – Area under T-s diagram = Heat Transfer (Q) T OR T ds s – Reversible process – Specific entropy s , J/(kg K) 05 -Jun-07 8

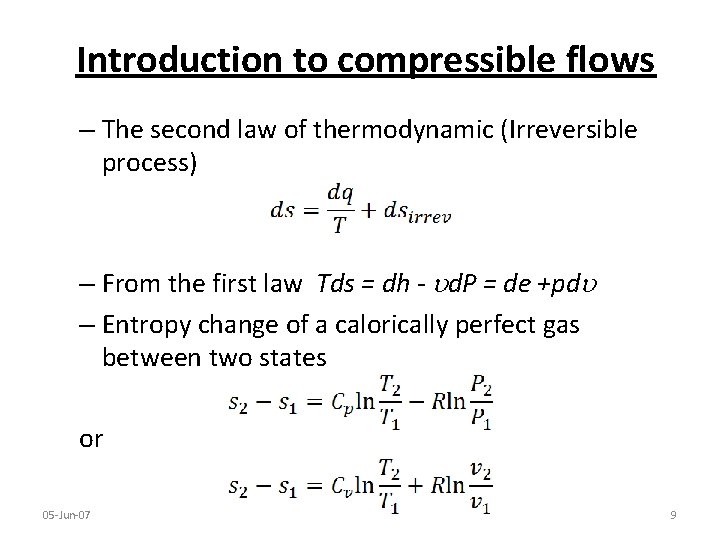
Introduction to compressible flows – The second law of thermodynamic (Irreversible process) – From the first law Tds = dh - d. P = de +pd – Entropy change of a calorically perfect gas between two states or 05 -Jun-07 9

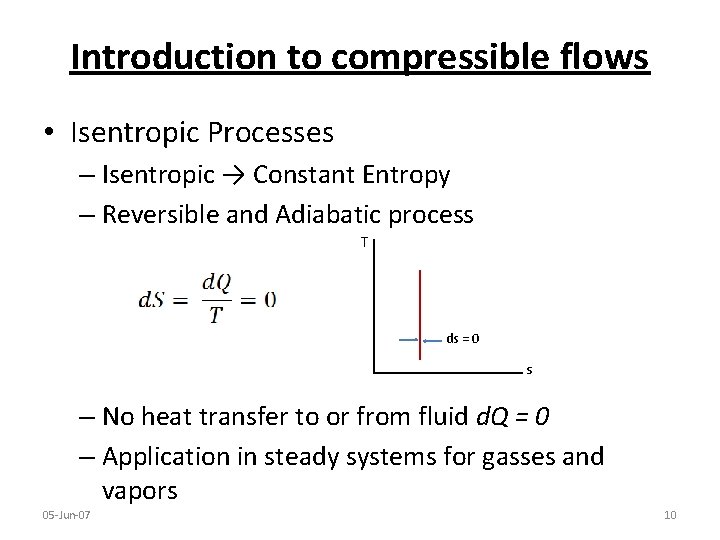
Introduction to compressible flows • Isentropic Processes – Isentropic → Constant Entropy – Reversible and Adiabatic process T ds = 0 s – No heat transfer to or from fluid d. Q = 0 – Application in steady systems for gasses and vapors 05 -Jun-07 10

Introduction to compressible flows • Exercise • 1) A perfect gas is expanded adiabatically from 5 to 1 bar by the law PV 1. 2 = Constant. The initial temperature is 200°C. Calculate the change in specific entropy. R = 287. 15 J/kg. K, =1. 4 05 -Jun-07 11

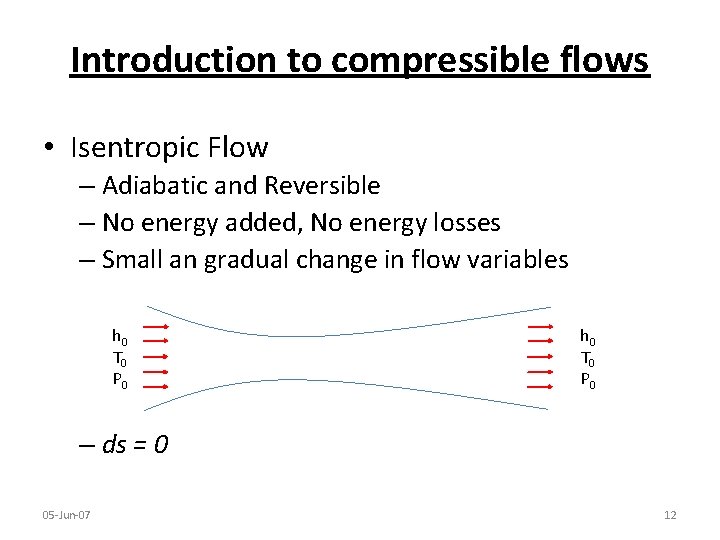
Introduction to compressible flows • Isentropic Flow – Adiabatic and Reversible – No energy added, No energy losses – Small an gradual change in flow variables h 0 T 0 P 0 – ds = 0 05 -Jun-07 12

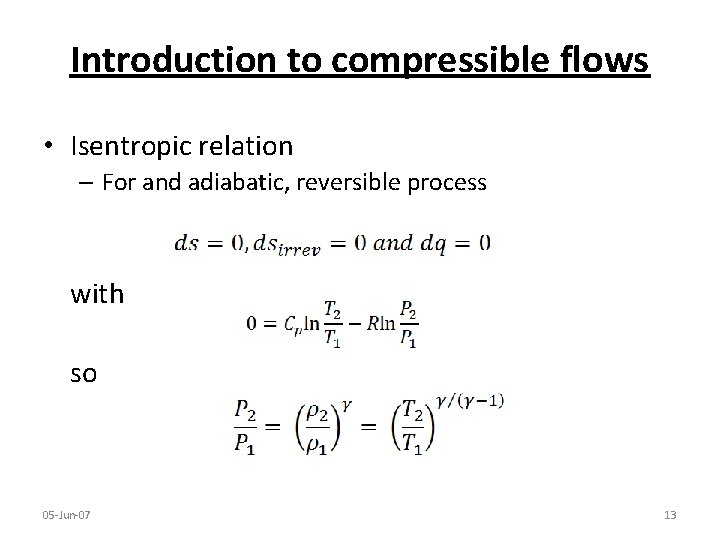
Introduction to compressible flows • Isentropic relation – For and adiabatic, reversible process with so 05 -Jun-07 13

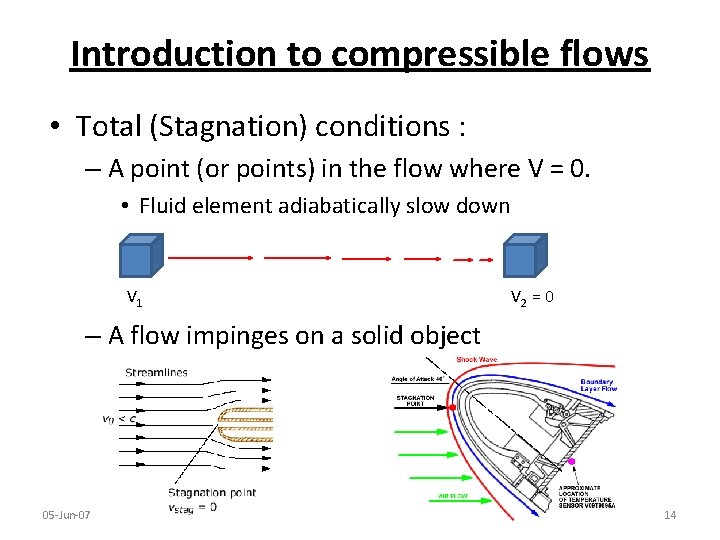
Introduction to compressible flows • Total (Stagnation) conditions : – A point (or points) in the flow where V = 0. • Fluid element adiabatically slow down V 1 V 2 = 0 – A flow impinges on a solid object 05 -Jun-07 14

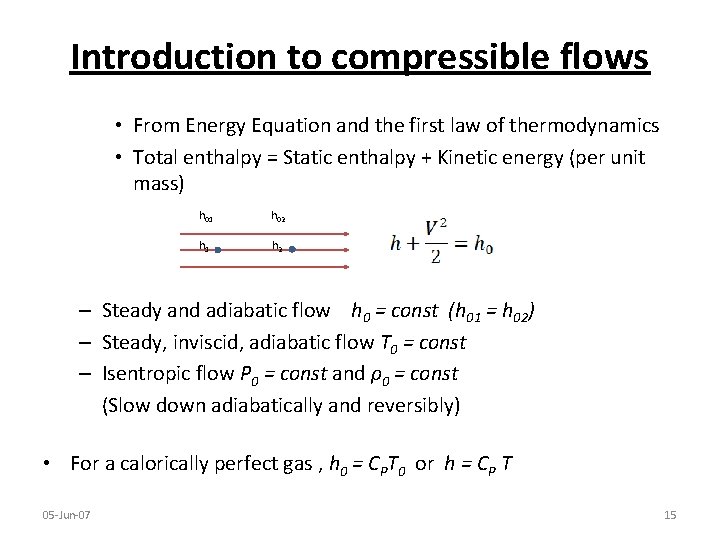
Introduction to compressible flows • From Energy Equation and the first law of thermodynamics • Total enthalpy = Static enthalpy + Kinetic energy (per unit mass) h 01 h 02 h 1 h 2 – Steady and adiabatic flow h 0 = const (h 01 = h 02) – Steady, inviscid, adiabatic flow T 0 = const – Isentropic flow P 0 = const and ρ0 = const (Slow down adiabatically and reversibly) • For a calorically perfect gas , h 0 = CPT 0 or h = CP T 05 -Jun-07 15

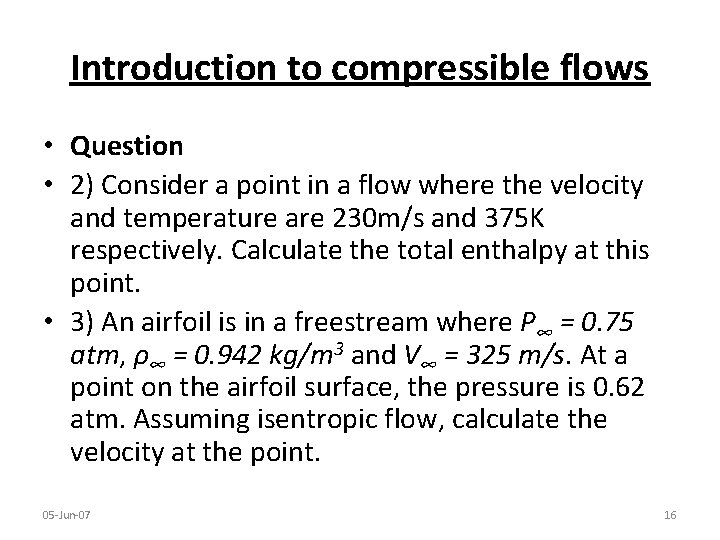
Introduction to compressible flows • Question • 2) Consider a point in a flow where the velocity and temperature are 230 m/s and 375 K respectively. Calculate the total enthalpy at this point. • 3) An airfoil is in a freestream where P∞ = 0. 75 atm, ρ∞ = 0. 942 kg/m 3 and V∞ = 325 m/s. At a point on the airfoil surface, the pressure is 0. 62 atm. Assuming isentropic flow, calculate the velocity at the point. 05 -Jun-07 16

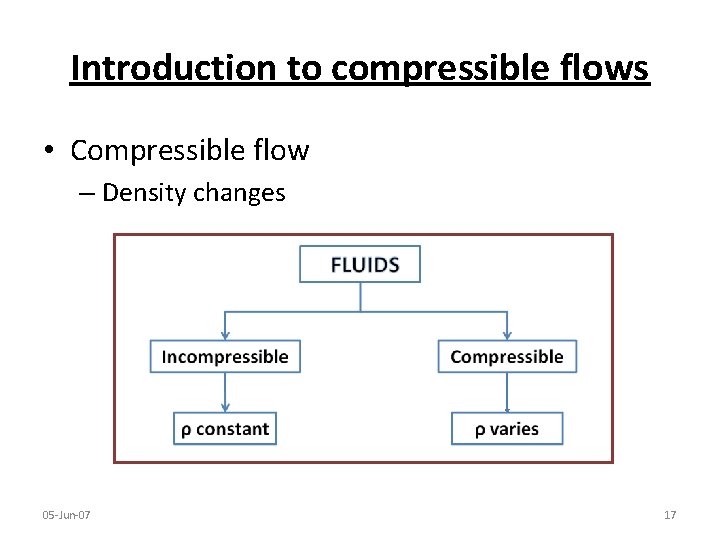
Introduction to compressible flows • Compressible flow – Density changes 05 -Jun-07 17

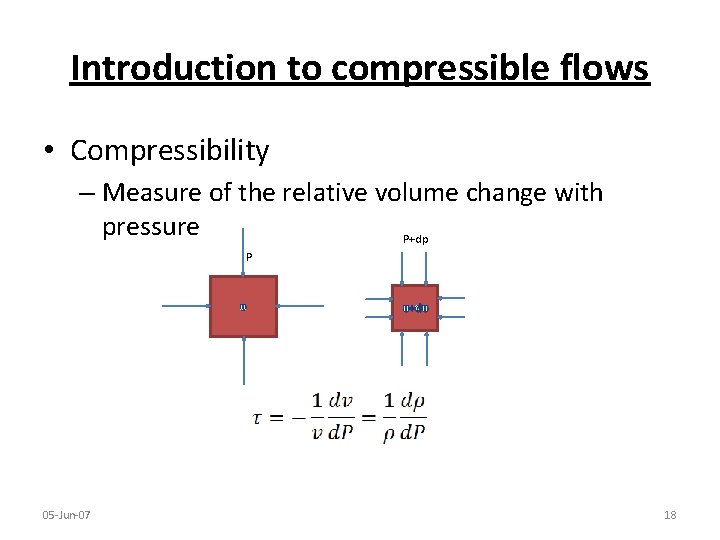
Introduction to compressible flows • Compressibility – Measure of the relative volume change with pressure P+dp P υ 05 -Jun-07 υ+dυ 18

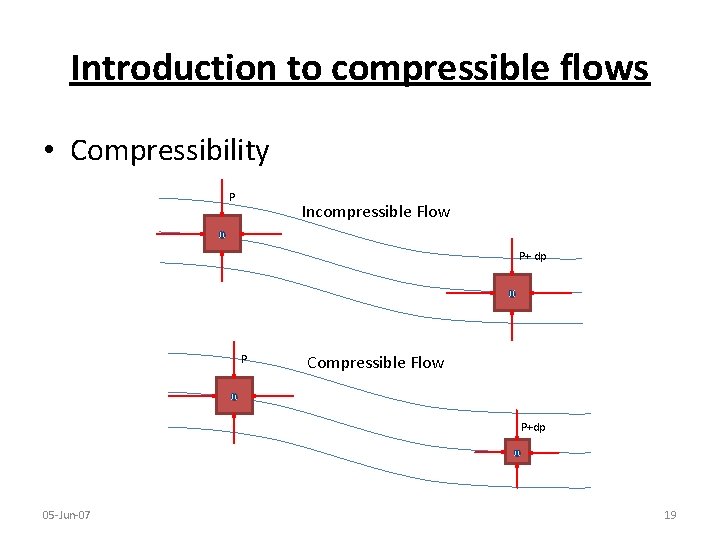
Introduction to compressible flows • Compressibility P Incompressible Flow υ P+ dp υ P Compressible Flow υ P+dp υ 05 -Jun-07 19

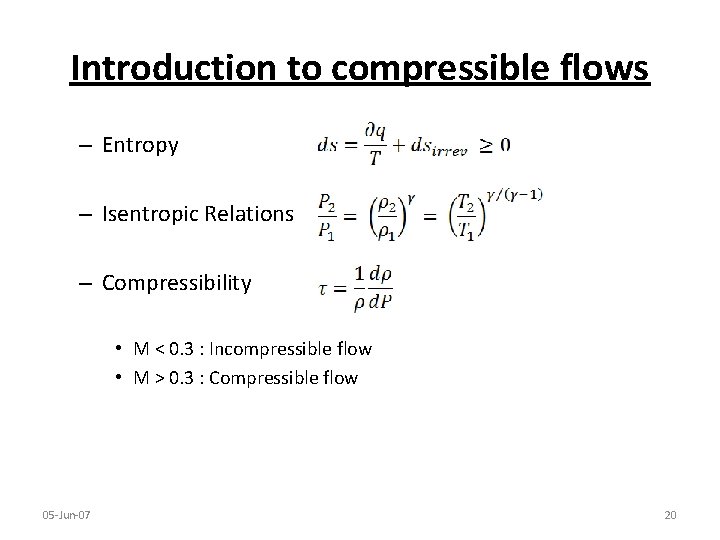
Introduction to compressible flows – Entropy – Isentropic Relations – Compressibility • M < 0. 3 : Incompressible flow • M > 0. 3 : Compressible flow 05 -Jun-07 20