High Risk MDS and Novel Therapy Whats on














- Slides: 47

High Risk MDS and Novel Therapy: What’s on the Horizon? Rafael Bejar MD, Ph. D Aplastic Anemia & MDS International Foundation Regional Patient and Family Conference April 5 th, 2014

Overview • Refining Prognosis and ‘High’ Risk • Novel Treatments • Advances in Stem Cell Transplantation • Examples from the Lab

Refining Prognosis

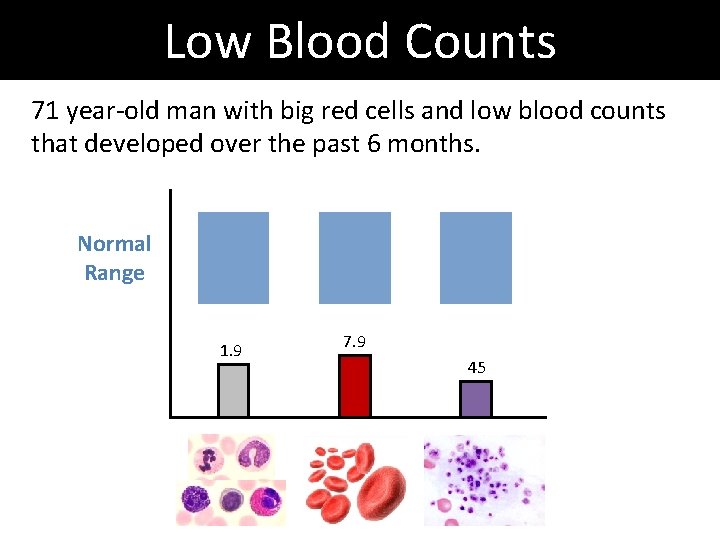
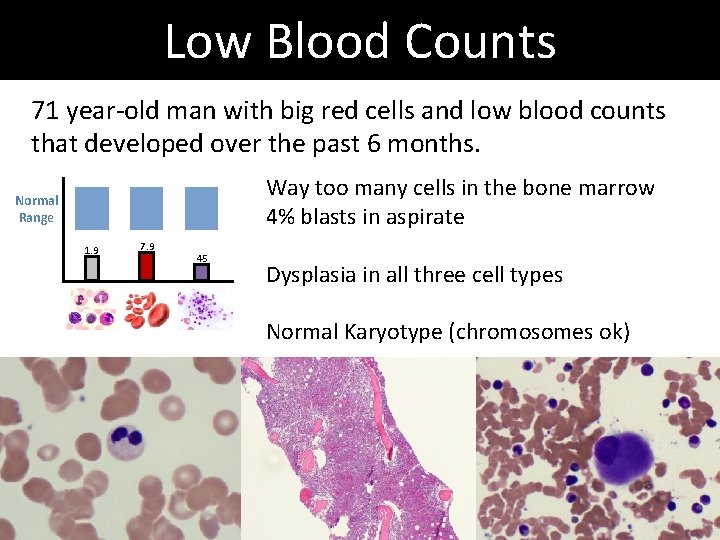
Low Blood Counts 71 year-old man with big red cells and low blood counts that developed over the past 6 months. Normal Range 1. 9 7. 9 45

Low Blood Counts 71 year-old man with big red cells and low blood counts that developed over the past 6 months. Way too many cells in the bone marrow 4% blasts in aspirate Normal Range 1. 9 7. 9 45 Dysplasia in all three cell types Normal Karyotype (chromosomes ok)

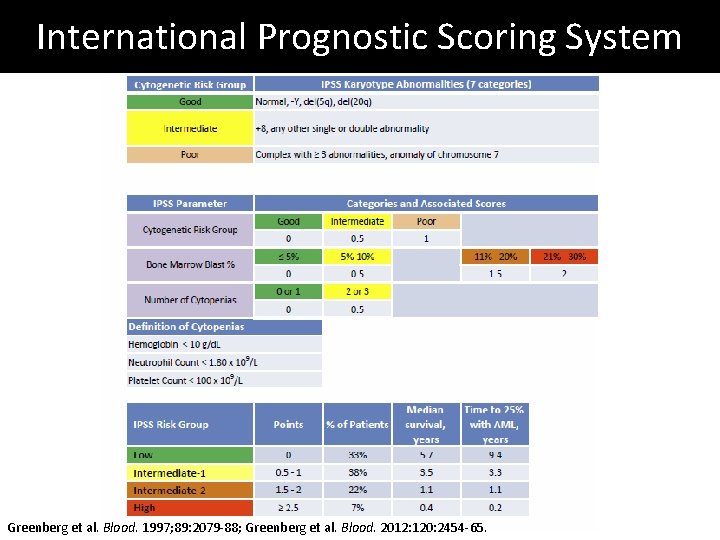
International Prognostic Scoring System Greenberg et al. Blood. 1997; 89: 2079 -88; Greenberg et al. Blood. 2012: 120: 2454 -65.

MDA Lower Risk Model LR-PSS Prognostic Score Value Prognostic Category 1 2 Risk Category Risk Score ≥ 60 1 0 -2 2 3 -4 3 ≥ 5 Not normal or del(5 q) Cytogenetics Age, years Hemoglobin, g/d. L < 10 Platelets, × 109/L 50 -200 < 50 ≥ 4% BM blasts, % 100 25%-33% of patients are in Category 3 Patients, % 80 The survival of Category 3 patients is similar to that of Intermediate-2 risk patients using the IPSS! 60 40 20 0 0 12 24 36 48 60 72 84 Survival, months from referral 96 Garcia-Manero G, et al. Leukemia. 2008; 22: 538 -543.

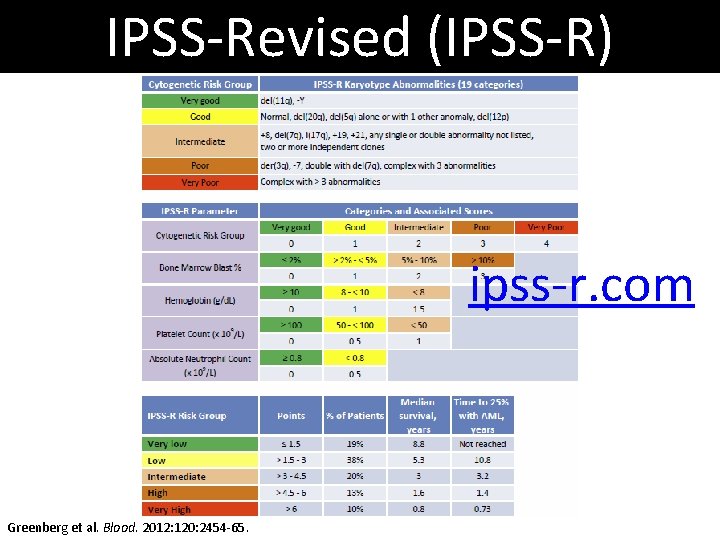
IPSS-Revised (IPSS-R) ipss-r. com Greenberg et al. Blood. 2012: 120: 2454 -65.

Current Therapies

Guidelines for Higher Risk MDS Goal: to improve DURATION OF LIFE

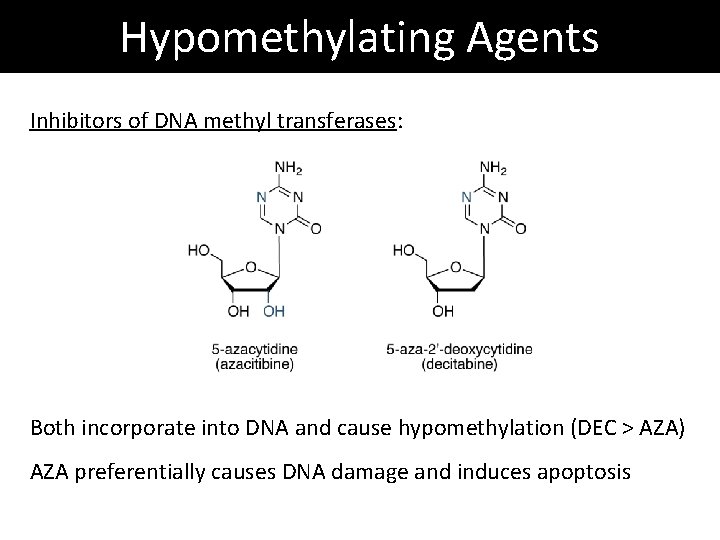
Hypomethylating Agents Inhibitors of DNA methyl transferases: Both incorporate into DNA and cause hypomethylation (DEC > AZA) AZA preferentially causes DNA damage and induces apoptosis

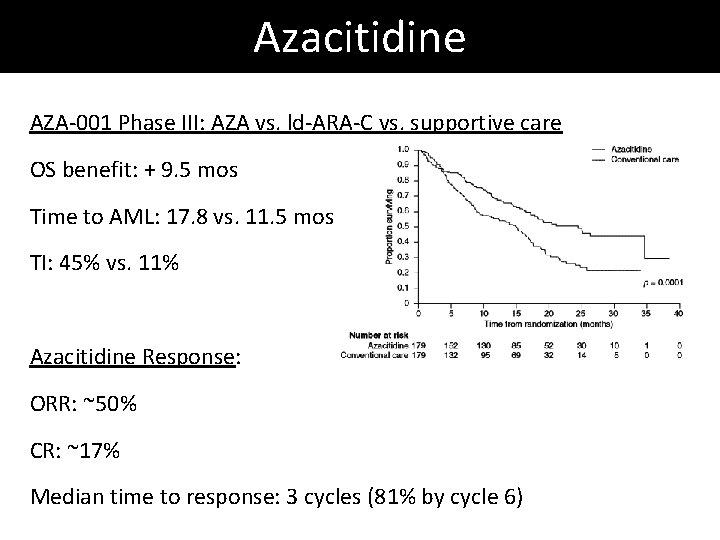
Azacitidine AZA-001 Phase III: AZA vs. ld-ARA-C vs. supportive care OS benefit: + 9. 5 mos Time to AML: 17. 8 vs. 11. 5 mos TI: 45% vs. 11% Azacitidine Response: ORR: ~50% CR: ~17% Median time to response: 3 cycles (81% by cycle 6)

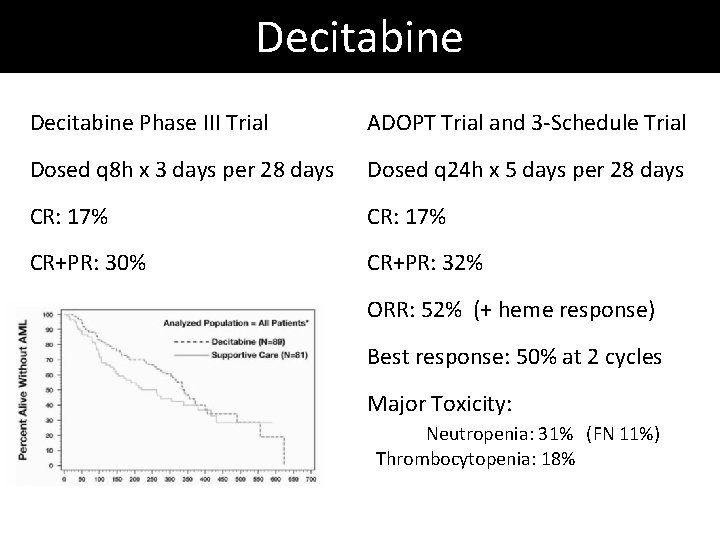
Decitabine Phase III Trial ADOPT Trial and 3 -Schedule Trial Dosed q 8 h x 3 days per 28 days Dosed q 24 h x 5 days per 28 days CR: 17% CR+PR: 30% CR+PR: 32% ORR: 52% (+ heme response) Best response: 50% at 2 cycles Major Toxicity: Neutropenia: 31% (FN 11%) Thrombocytopenia: 18%

Guidelines for Higher Risk MDS Goal: to improve DURATION OF LIFE Special Considerations: Refer for Transplant Early - Even patients in their 70’s can benefit from RIC transplant AZA > DEC (for now) - AZA has been shown to have a survival advantage, DEC has not (yet) Don’t forget Quality of Life - Consider treatment palliative and weigh against patient needs Look for Clinical Trials - Few option after AZA are available and none are approved

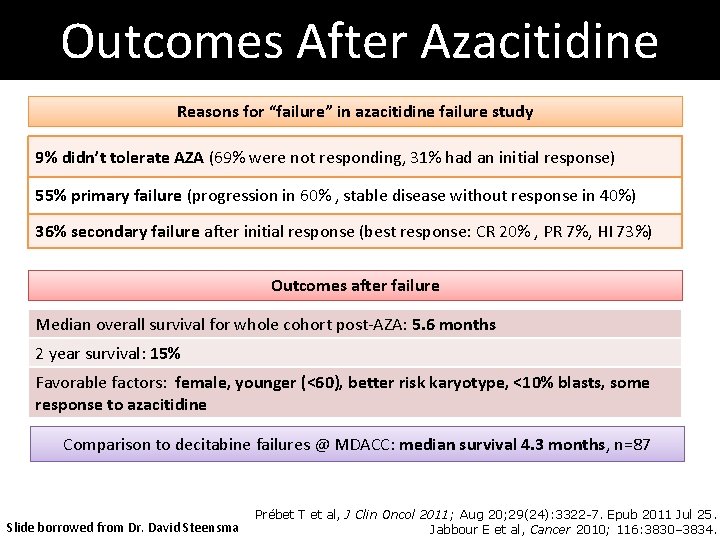
Outcomes After Azacitidine Reasons for “failure” in azacitidine failure study 9% didn’t tolerate AZA (69% were not responding, 31% had an initial response) 55% primary failure (progression in 60% , stable disease without response in 40%) 36% secondary failure after initial response (best response: CR 20% , PR 7%, HI 73%) Outcomes after failure Median overall survival for whole cohort post-AZA: 5. 6 months 2 year survival: 15% Favorable factors: female, younger (<60), better risk karyotype, <10% blasts, some response to azacitidine Comparison to decitabine failures @ MDACC: median survival 4. 3 months, n=87 Slide borrowed from Dr. David Steensma Prébet T et al, J Clin Oncol 2011; Aug 20; 29(24): 3322 -7. Epub 2011 Jul 25. Jabbour E et al, Cancer 2010; 116: 3830– 3834.

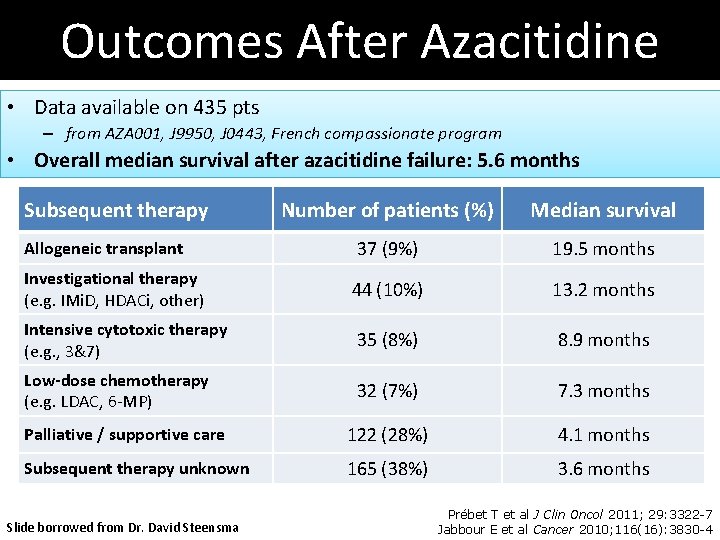
Outcomes After Azacitidine • Data available on 435 pts – from AZA 001, J 9950, J 0443, French compassionate program • Overall median survival after azacitidine failure: 5. 6 months Subsequent therapy Number of patients (%) Median survival Allogeneic transplant 37 (9%) 19. 5 months Investigational therapy (e. g. IMi. D, HDACi, other) 44 (10%) 13. 2 months Intensive cytotoxic therapy (e. g. , 3&7) 35 (8%) 8. 9 months Low-dose chemotherapy (e. g. LDAC, 6 -MP) 32 (7%) 7. 3 months Palliative / supportive care 122 (28%) 4. 1 months Subsequent therapy unknown 165 (38%) 3. 6 months Slide borrowed from Dr. David Steensma Prébet T et al J Clin Oncol 2011; 29: 3322 -7 Jabbour E et al Cancer 2010; 116(16): 3830 -4

Treatment of Higher Risk MDS We need BETTER therapies! We need MORE therapies!

Better Formulations

Oral Azacitidine 2011 – Oral AZA given 7 days out of 28 is safe and appears effective 2012 – Treating for 14 or 21 days enhances biologic activity and is effective – 34% ORR and 40% transfusion independent 2013 – Phase III Clinical Trial of Lower Risk Transfusion Dependence - should lead to FDA approval PROS Oral drug that can be taken at home CONS Gastrointestinal side effects May take 6 -8 cycles to reach maximum response

SGI-110 Resistant to degradation Longer half-life May allow less frequent dosing - 5 days vs. once weekly In clinical trials at USC Yoo C B et al. Cancer Res 2007; 67: 6400 -8.

Combination Therapies

Combination Therapy Approach: Improve upon existing therapies Example: At least 2 clinic trials in development combine: Azacitidine + Deferasirox (Exjade) Advantage: drugs are already FDA approved for MDS Positive results can quickly change practice!

Lenalidomide + Azacitidine • Multicenter, single-arm open-label phase II continuation study (N = 36) • Patient eligibility – Higher-risk MDS: CMML-2, RAEB-1 or -2, IPSS intermediate 2 or high (score ≥ 1. 5), or revised IPSS score 4 or 5 – No previous treatment with lenalidomide or azacitidine • Maximum of seven 28 -day treatment cycles administered – Lenalidomide 10 mg on Days 1 -21 – Azacitidine 75 mg/m 2 on Days 1 -5 – After 7 cycles, patients could continue azacitidine monotherapy off study • Median patient follow-up: 12 mos (range: 3 -55) Slide borrowed from Dr. Rami Komrokji Sekeres MA, et al. Blood. 2012; 120: 4945 -4951.

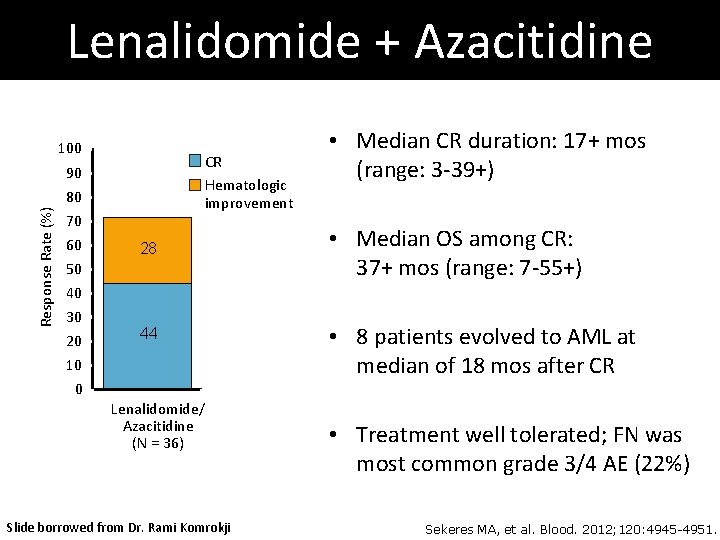
Lenalidomide + Azacitidine 100 CR Hematologic improvement Response Rate (%) 90 80 70 60 28 50 40 30 20 10 0 44 Lenalidomide/ Azacitidine (N = 36) Slide borrowed from Dr. Rami Komrokji • Median CR duration: 17+ mos (range: 3 -39+) • Median OS among CR: 37+ mos (range: 7 -55+) • 8 patients evolved to AML at median of 18 mos after CR • Treatment well tolerated; FN was most common grade 3/4 AE (22%) Sekeres MA, et al. Blood. 2012; 120: 4945 -4951.

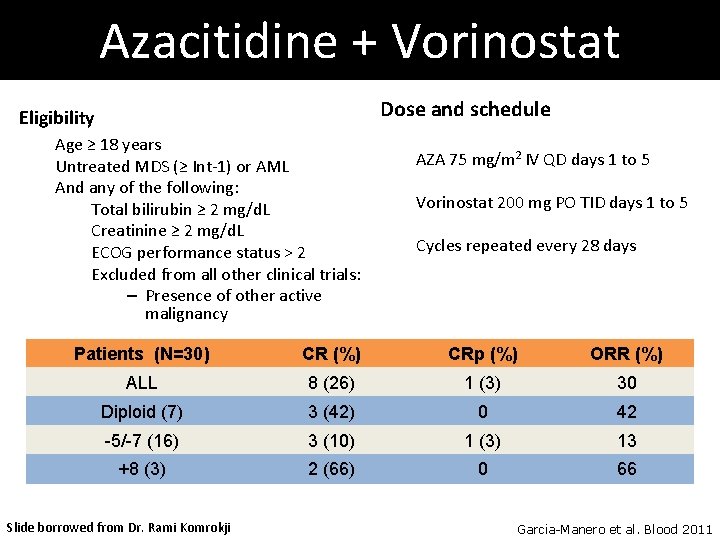
Azacitidine + Vorinostat Dose and schedule Eligibility Age ≥ 18 years Untreated MDS (≥ Int-1) or AML And any of the following: Total bilirubin ≥ 2 mg/d. L Creatinine ≥ 2 mg/d. L ECOG performance status > 2 Excluded from all other clinical trials: – Presence of other active malignancy AZA 75 mg/m 2 IV QD days 1 to 5 Vorinostat 200 mg PO TID days 1 to 5 Cycles repeated every 28 days Patients (N=30) CR (%) CRp (%) ORR (%) ALL 8 (26) 1 (3) 30 Diploid (7) 3 (42) 0 42 -5/-7 (16) 3 (10) 1 (3) 13 +8 (3) 2 (66) 0 66 Slide borrowed from Dr. Rami Komrokji Garcia-Manero et al. Blood 2011

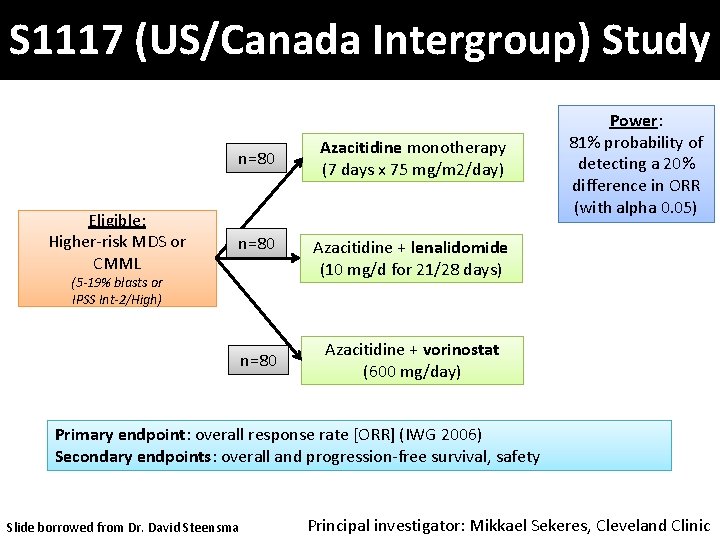
S 1117 (US/Canada Intergroup) Study n=80 Eligible: Higher-risk MDS or CMML n=80 (5 -19% blasts or IPSS Int-2/High) n=80 Azacitidine monotherapy (7 days x 75 mg/m 2/day) Power: 81% probability of detecting a 20% difference in ORR (with alpha 0. 05) Azacitidine + lenalidomide (10 mg/d for 21/28 days) Azacitidine + vorinostat (600 mg/day) Primary endpoint: overall response rate [ORR] (IWG 2006) Secondary endpoints: overall and progression-free survival, safety Slide borrowed from Dr. David Steensma Principal investigator: Mikkael Sekeres, Cleveland Clinic

Novel Agents

Pipeline of Completely New Drugs Ezatiostat MAP Kinase Inhibitors TGF-beta Inhibitors Suffering Neddylation Inhibitors Indolamine Dioxygenase Inhibitors p 53 Modulators Hedgehog Inhibitors Aminopeptidase Inhibitors RNA Pol I Inhibitors Options Anti-CD 47 Antibodies

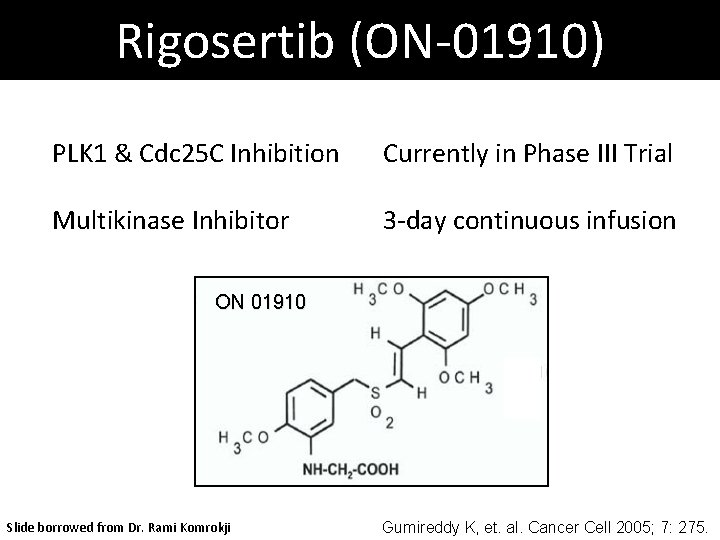
Rigosertib (ON-01910) PLK 1 & Cdc 25 C Inhibition Currently in Phase III Trial Multikinase Inhibitor 3 -day continuous infusion ON 01910 Slide borrowed from Dr. Rami Komrokji Gumireddy K, et. al. Cancer Cell 2005; 7: 275.

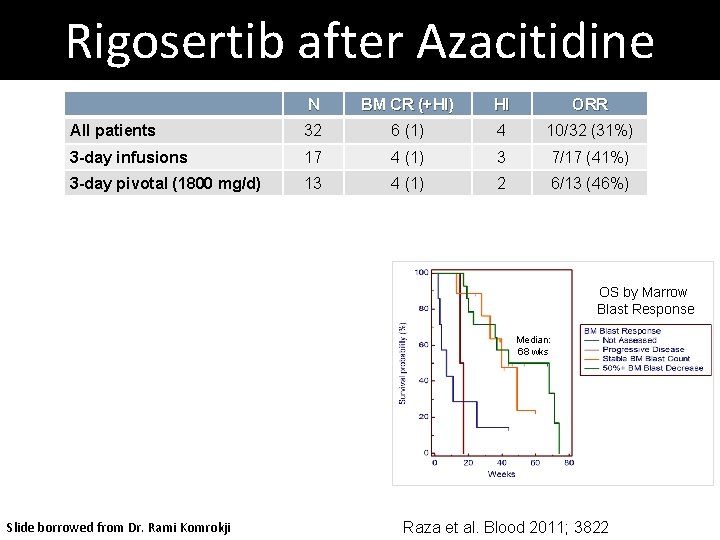
Rigosertib after Azacitidine N BM CR (+HI) HI ORR All patients 32 6 (1) 4 10/32 (31%) 3 -day infusions 17 4 (1) 3 7/17 (41%) 3 -day pivotal (1800 mg/d) 13 4 (1) 2 6/13 (46%) OS by Marrow Blast Response Median: 68 wks Slide borrowed from Dr. Rami Komrokji Raza et al. Blood 2011; 3822

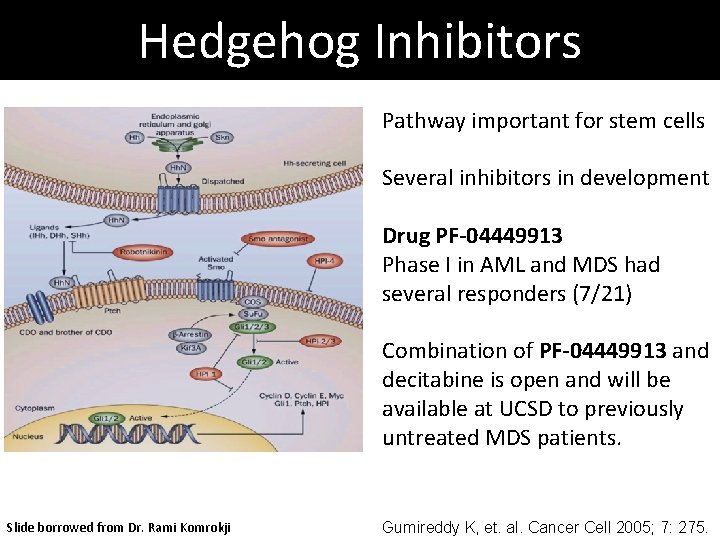
Hedgehog Inhibitors Pathway important for stem cells Several inhibitors in development Drug PF-04449913 Phase I in AML and MDS had several responders (7/21) Combination of PF-04449913 and decitabine is open and will be available at UCSD to previously untreated MDS patients. Slide borrowed from Dr. Rami Komrokji Gumireddy K, et. al. Cancer Cell 2005; 7: 275.

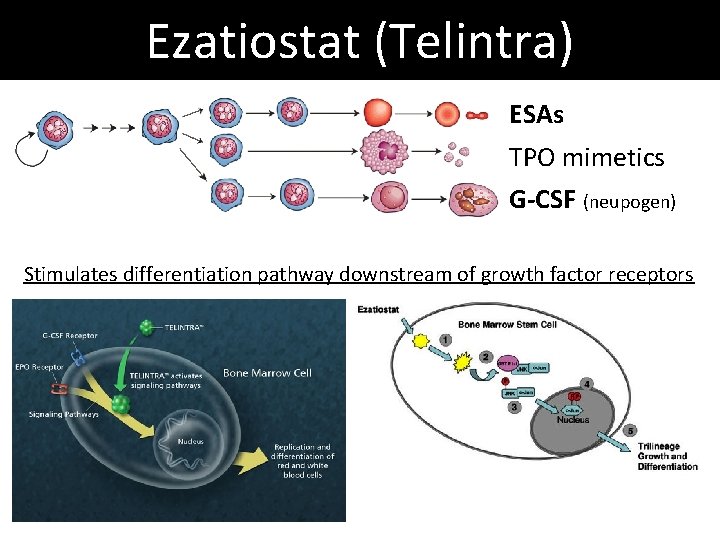
Ezatiostat (Telintra) ESAs TPO mimetics G-CSF (neupogen) Stimulates differentiation pathway downstream of growth factor receptors

Ezatiostat (Telintra) Target population are lower risk patients, but trials show activity in heavily pretreated patients! Phase II Study: - 56% had previously received Azacitidine or Decitabine - 38% had previously received Lenalidomide - 77% had previously received Erythropoietin Results: - 22% had a red cell response - 19% had a neutrophil response - 20% had both - 29% had reduced transfusion needs - average time to response was 8 WEEKS! - very little toxicity!! Raza et al. Cancer. 2013. 118(8) 2138 -47.

Stem Cell Transplantation

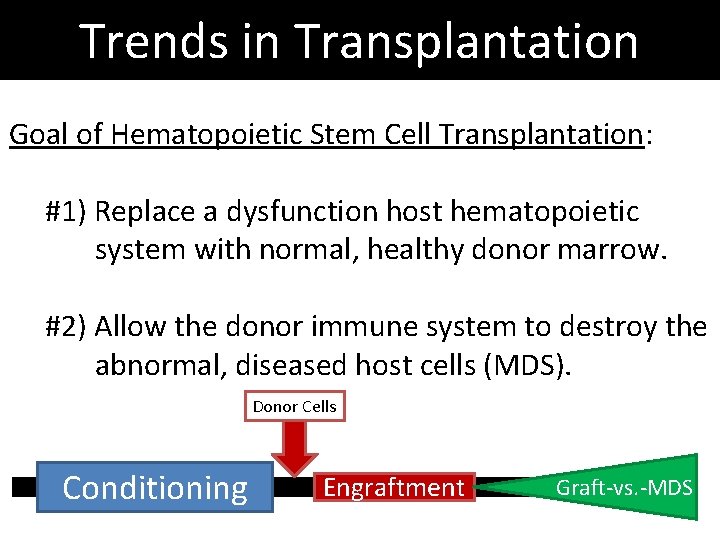
Trends in Transplantation Goal of Hematopoietic Stem Cell Transplantation: #1) Replace a dysfunction host hematopoietic system with normal, healthy donor marrow. #2) Allow the donor immune system to destroy the abnormal, diseased host cells (MDS). Donor Cells Conditioning Engraftment Graft-vs. -MDS

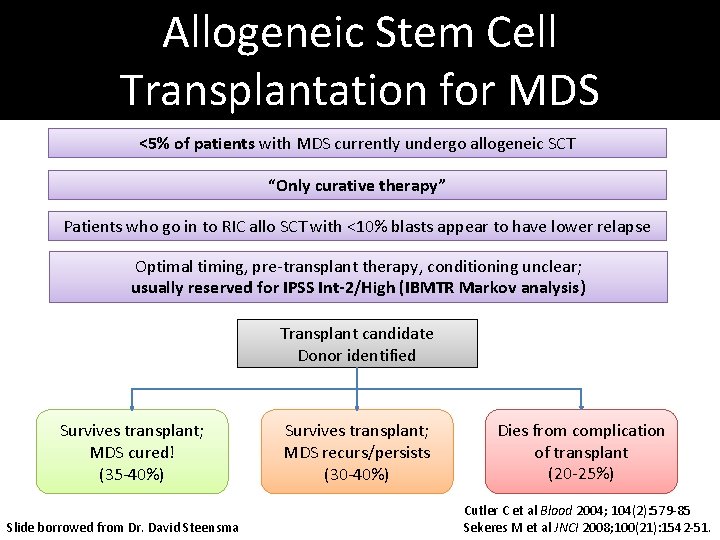
Allogeneic Stem Cell Transplantation for MDS <5% of patients with MDS currently undergo allogeneic SCT “Only curative therapy” Patients who go in to RIC allo SCT with <10% blasts appear to have lower relapse Optimal timing, pre-transplant therapy, conditioning unclear; usually reserved for IPSS Int-2/High (IBMTR Markov analysis) Transplant candidate Donor identified Survives transplant; MDS cured! (35 -40%) Slide borrowed from Dr. David Steensma Survives transplant; MDS recurs/persists (30 -40%) Dies from complication of transplant (20 -25%) Cutler C et al Blood 2004; 104(2): 579 -85 Sekeres M et al JNCI 2008; 100(21): 1542 -51.

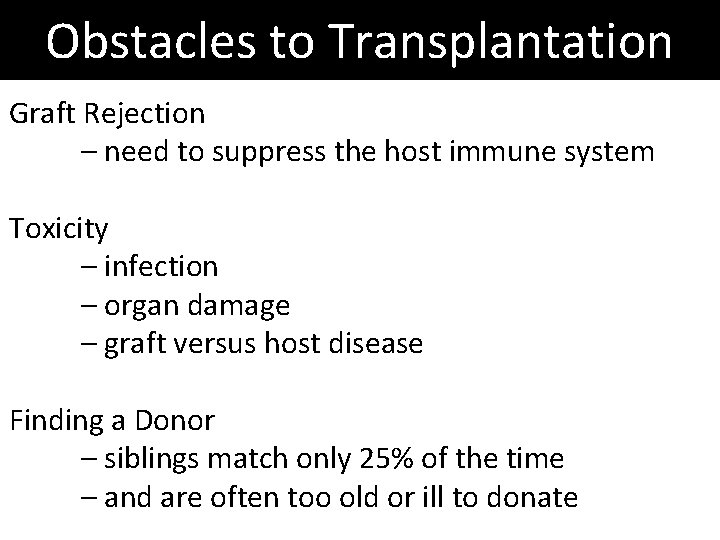
Obstacles to Transplantation Graft Rejection – need to suppress the host immune system Toxicity – infection – organ damage – graft versus host disease Finding a Donor – siblings match only 25% of the time – and are often too old or ill to donate

Overcoming Obstacles Avoiding Graft Rejection – better approaches to immune suppression Less Toxicity – better supportive care – better antigen matching – reduced intensity conditioning Alternative Sources for Stem Cells – haploidentical – “half” match – umbilical cord blood stem cells


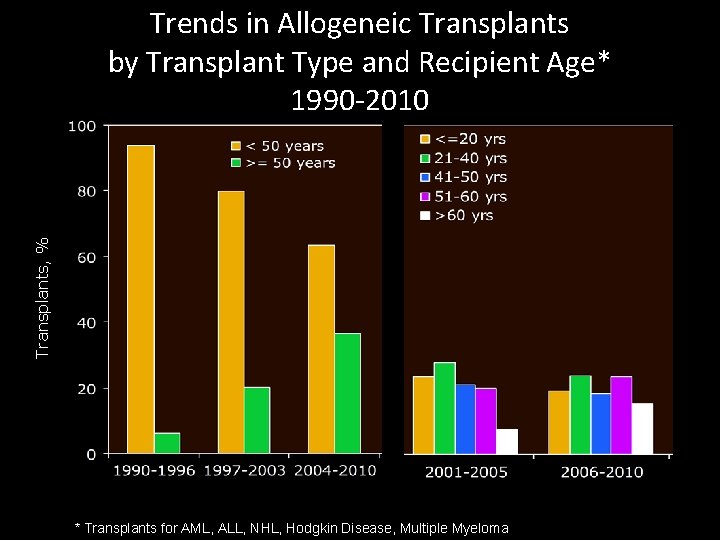
Transplants, % Trends in Allogeneic Transplants by Transplant Type and Recipient Age* 1990 -2010 * Transplants for AML, ALL, NHL, Hodgkin Disease, Multiple Myeloma

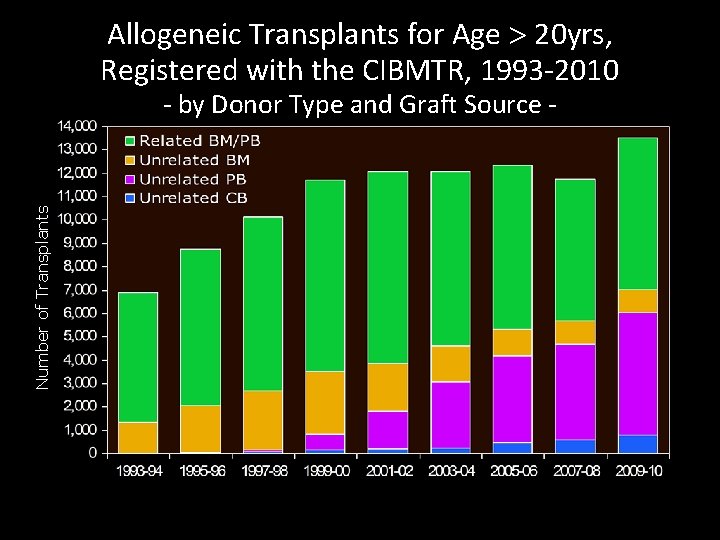
Allogeneic Transplants for Age > 20 yrs, Registered with the CIBMTR, 1993 -2010 Number of Transplants - by Donor Type and Graft Source -

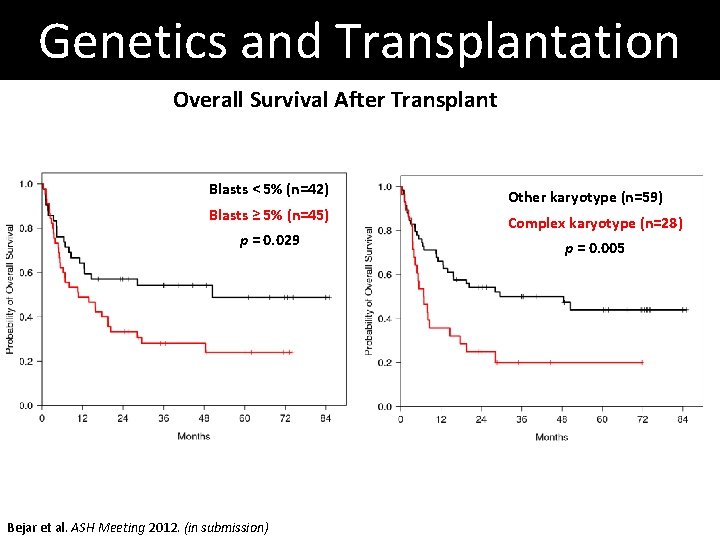
Genetics and Transplantation Overall Survival After Transplant Blasts < 5% (n=42) Blasts ≥ 5% (n=45) p = 0. 029 Bejar et al. ASH Meeting 2012. (in submission) Other karyotype (n=59) Complex karyotype (n=28) p = 0. 005

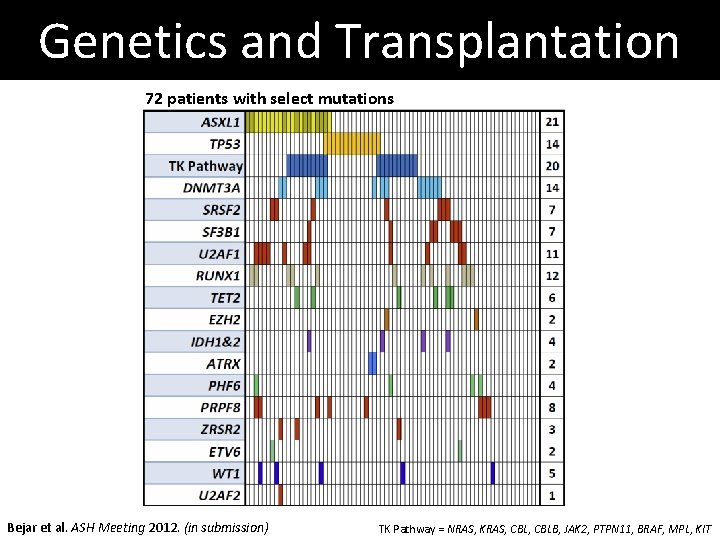
Genetics and Transplantation 72 patients with select mutations Bejar et al. ASH Meeting 2012. (in submission) TK Pathway = NRAS, KRAS, CBLB, JAK 2, PTPN 11, BRAF, MPL, KIT

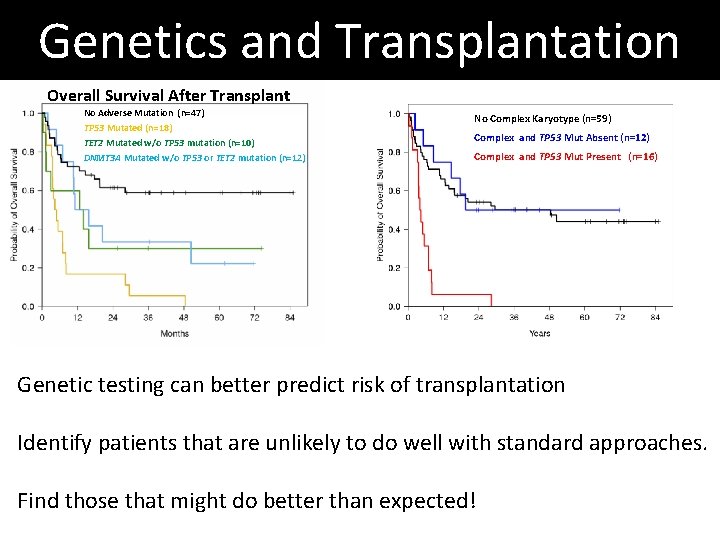
Genetics and Transplantation Overall Survival After Transplant No Adverse Mutation (n=47) TP 53 Mutated (n=18) TET 2 Mutated w/o TP 53 mutation (n=10) DNMT 3 A Mutated w/o TP 53 or TET 2 mutation (n=12) No Complex Karyotype (n=59) Complex and TP 53 Mut Absent (n=12) Complex and TP 53 Mut Present (n=16) Genetic testing can better predict risk of transplantation Identify patients that are unlikely to do well with standard approaches. Find those that might do better than expected!

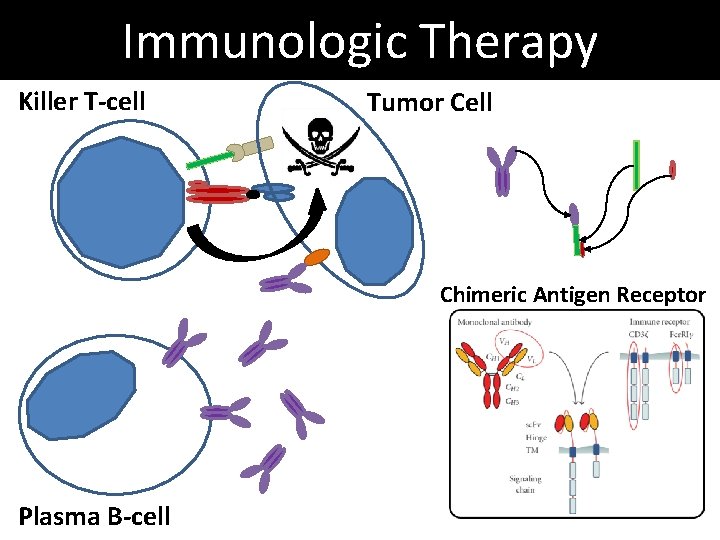
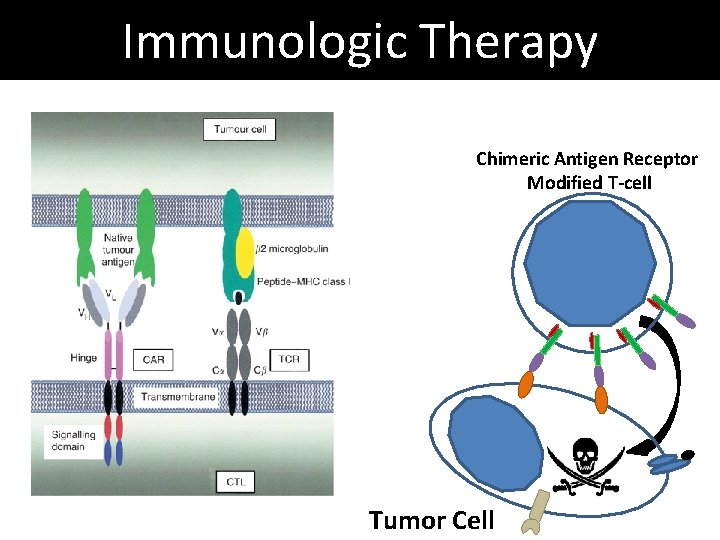
Immunologic Therapy Killer T-cell Tumor Cell Chimeric Antigen Receptor Plasma B-cell

Immunologic Therapy Chimeric Antigen Receptor Modified T-cell Tumor Cell

Acknowledgements: Bejar Lab - UCSD Columbia University Albert Perez Azra Raza Brigham and Women’s MD Anderson Cancer Center Ben Ebert Allegra Lord Ann Mullally Anu Narla Bennett Caughey Bernd Boidol Damien Wilpitz Marie Mc. Conkey Guillermo Garcia-Manero Hagop Kantarjian Sherry Pierce Gautam Borthakur DFCI / Broad David Steensma Donna Neuberg Kristen Stevenson Mike Makrigiorgos Derek Murphy Naomi Galili Memorial Sloan-Kettering Ross Levine Omar Abdel-Wahab