High Resolution Spectroscopy The Chemists Friend Chemistry of















- Slides: 44

High Resolution Spectroscopy: The Chemist's Friend

Chemistry of Hydrocarbon Oxidation Energy Environment Combustion Atmospheric Chemistry

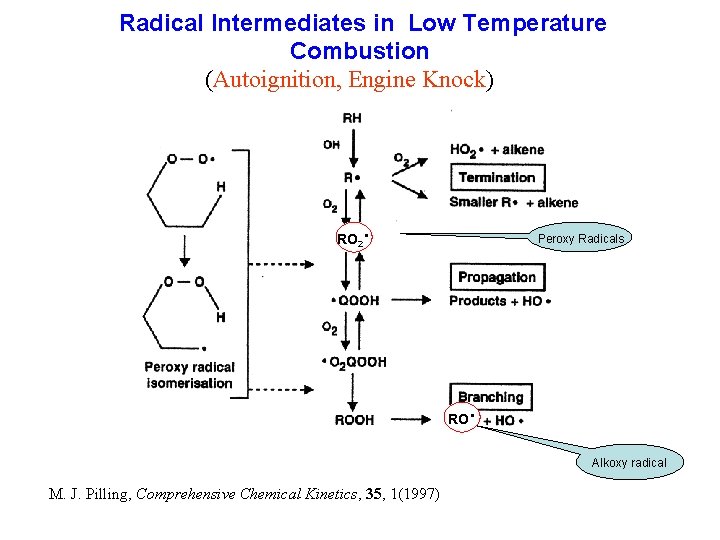
Radical Intermediates in Low Temperature Combustion (Autoignition, Engine Knock) · RO 2 Peroxy Radicals RO · Alkoxy radical M. J. Pilling, Comprehensive Chemical Kinetics, 35, 1(1997)

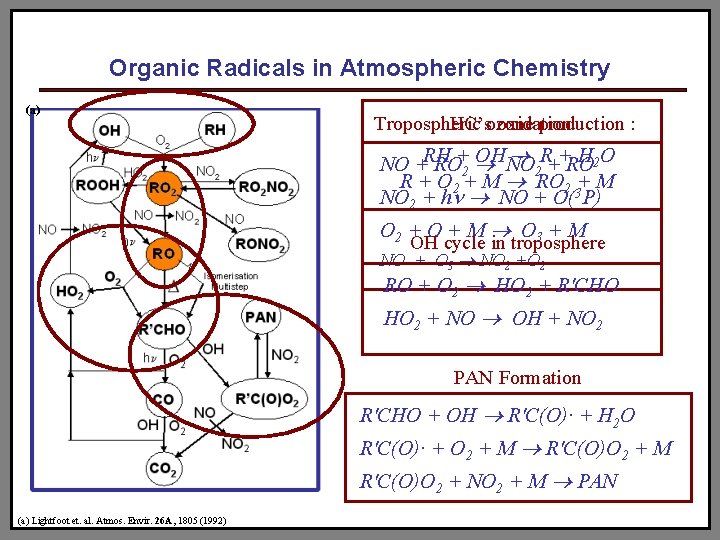
Organic Radicals in Atmospheric Chemistry (a) Tropospheric HC’sozone oxidation production : OHNO R++RO H 2 O NO +RH RO+2 2 R + O 2 + M RO 2 3+ M NO 2 + hn NO + O( P) O 2 + O + M O 3 + M OH cycle in troposphere NO + O 3 NO 2 +O 2 RO + O 2 HO 2 + R'CHO HO 2 + NO OH + NO 2 PAN Formation R'CHO + OH R'C(O)· + H 2 O R'C(O)· + O 2 + M R'C(O)O 2 + M R'C(O)O 2 + NO 2 + M PAN (a) Lightfoot et. al. Atmos. Envir. 26 A, 1805 (1992)

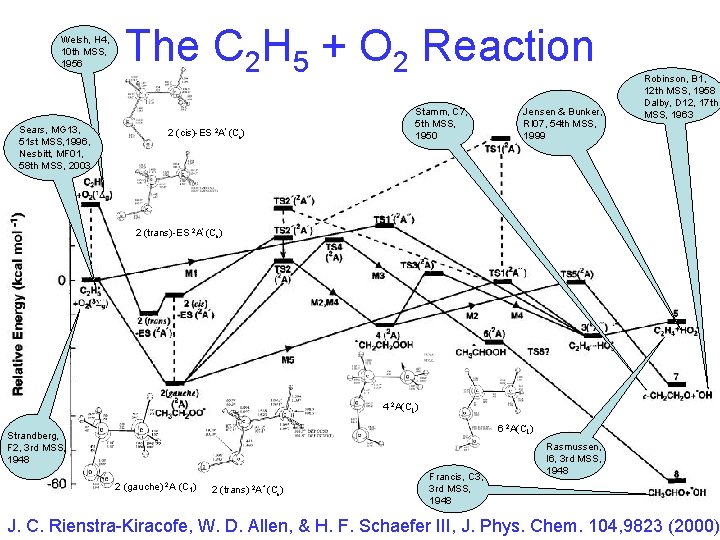
Welsh, H 4, 10 th MSS, 1956 Sears, MG 13, 51 st MSS, 1996, Nesbitt, MF 01, 58 th MSS, 2003 The C 2 H 5 + O 2 Reaction Stamm, C 7, 5 th MSS, 1950 2 (cis)-ES 2 A' (Cs) Jensen & Bunker, RI 07, 54 th MSS, 1999 Robinson, B 1, 12 th MSS, 1958 Dalby, D 12, 17 th MSS, 1963 2 (trans)-ES 2 A' (Cs) 4 2 A(C 1) 6 2 A(C 1) Strandberg, F 2, 3 rd MSS, 1948 2 (gauche) 2 A (C 1) 2 (trans) 2 A" (Cs) Francis, C 3, 3 rd MSS, 1948 Rasmussen, I 6, 3 rd MSS, 1948 J. C. Rienstra-Kiracofe, W. D. Allen, & H. F. Schaefer III, J. Phys. Chem. 104, 9823 (2000)

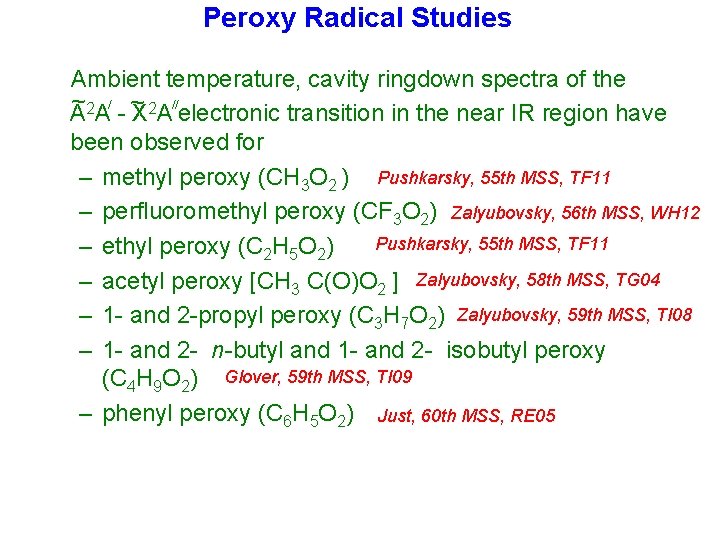
Peroxy Radical Studies Ambient temperature, cavity ringdown spectra of the ~2 / ~ 2 // A A - X A electronic transition in the near IR region have been observed for – methyl peroxy (CH 3 O 2 ) Pushkarsky, 55 th MSS, TF 11 – perfluoromethyl peroxy (CF 3 O 2) Zalyubovsky, 56 th MSS, WH 12 Pushkarsky, 55 th MSS, TF 11 – ethyl peroxy (C 2 H 5 O 2) – acetyl peroxy [CH 3 C(O)O 2 ] Zalyubovsky, 58 th MSS, TG 04 – 1 - and 2 -propyl peroxy (C 3 H 7 O 2) Zalyubovsky, 59 th MSS, TI 08 – 1 - and 2 - n-butyl and 1 - and 2 - isobutyl peroxy (C 4 H 9 O 2) Glover, 59 th MSS, TI 09 – phenyl peroxy (C 6 H 5 O 2) Just, 60 th MSS, RE 05

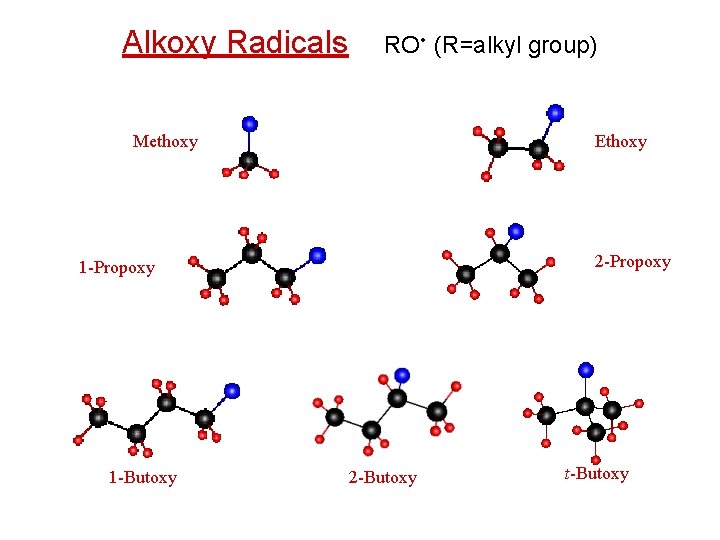
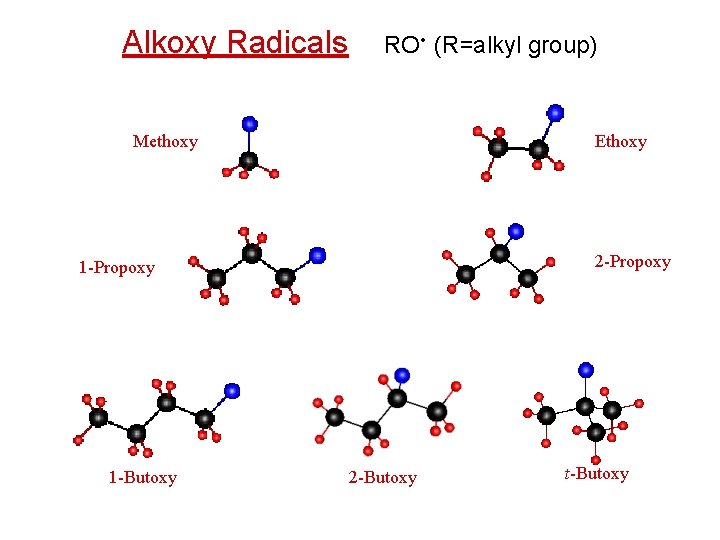
Alkoxy Radicals RO • (R=alkyl group) Methoxy Ethoxy 2 -Propoxy 1 -Butoxy 2 -Butoxy t-Butoxy

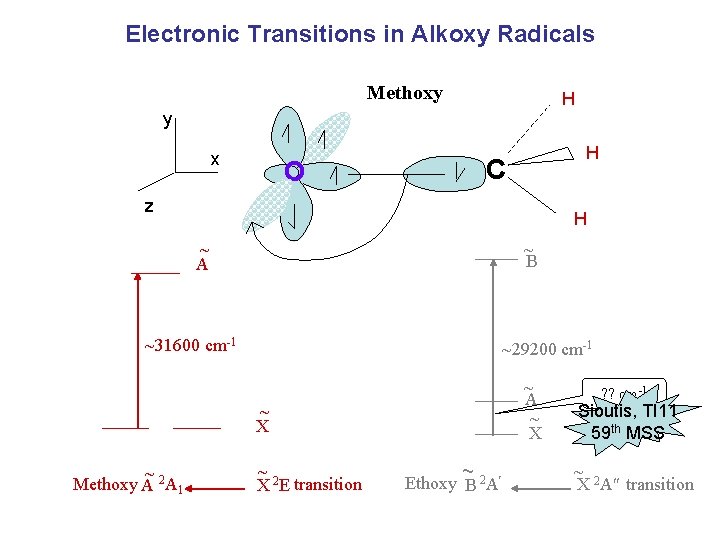
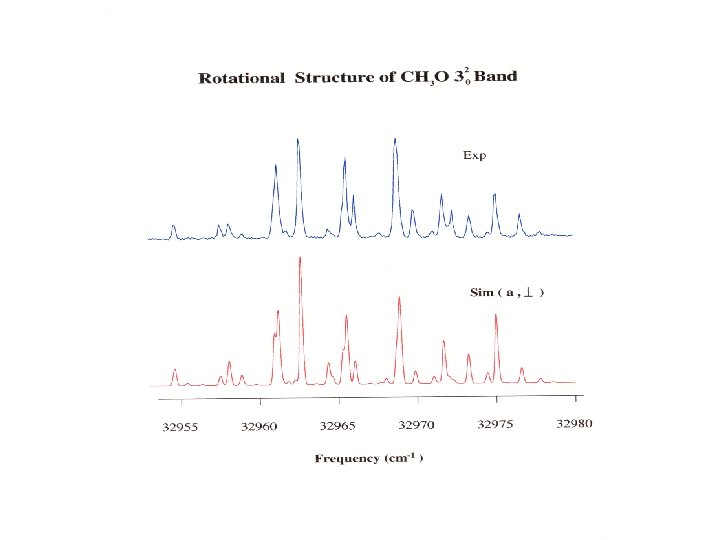
Electronic Transitions in Alkoxy Radicals Methoxy H y x O H C z H ~ A ~ B ~31600 cm-1 ~29200 cm-1 ~ A ~ X ~ Methoxy A 2 A 1 ~ X 2 E transition ~ Ethoxy B 2 A ? ? cm-1 Sioutis, TI 11 59 th MSS ~ 2 X A transition

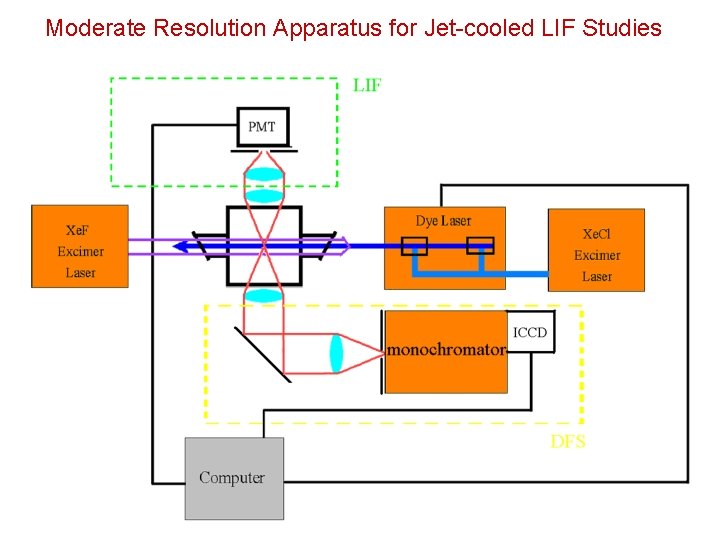
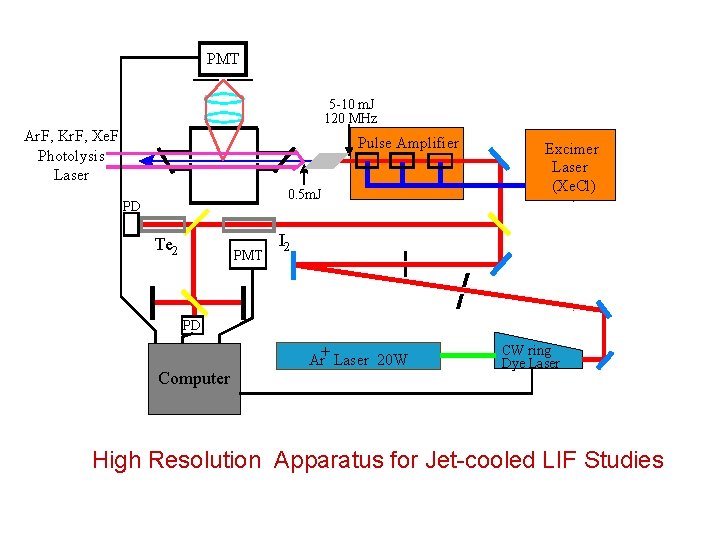
Moderate Resolution Apparatus for Jet-cooled LIF Studies

PMT 5 -10 m. J 120 MHz Ar. F, Kr. F, Xe. F Photolysis Laser Pulse Amplifier 0. 5 m. J PD Te 2 PMT Excimer Laser (Xe. Cl) I 2 PD Ar+ Laser 20 W Computer CW ring Dye Laser High Resolution Apparatus for Jet-cooled LIF Studies




Vibronically Resolved Spectrum of 2 -propoxy Free Jet Room Temperature Balla, Nelson, and Mc. Donald, Chem. Phys. 99, 323 (1985).

Alkoxy Radicals RO • (R=alkyl group) Methoxy Ethoxy 2 -Propoxy 1 -Butoxy 2 -Butoxy t-Butoxy

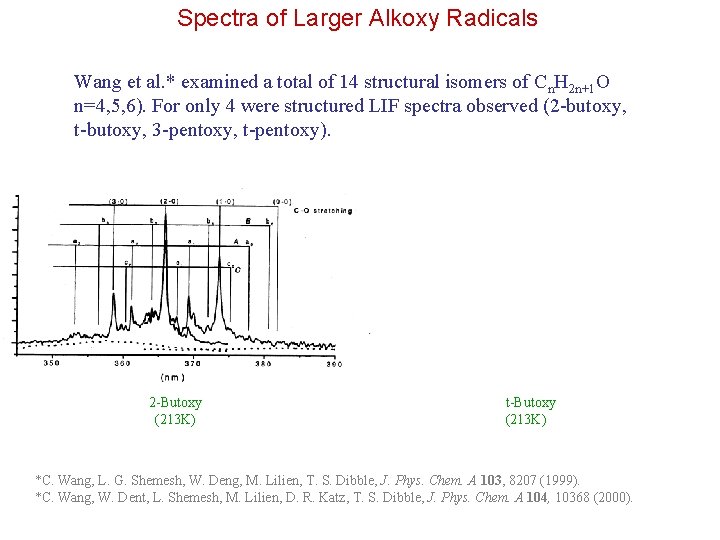
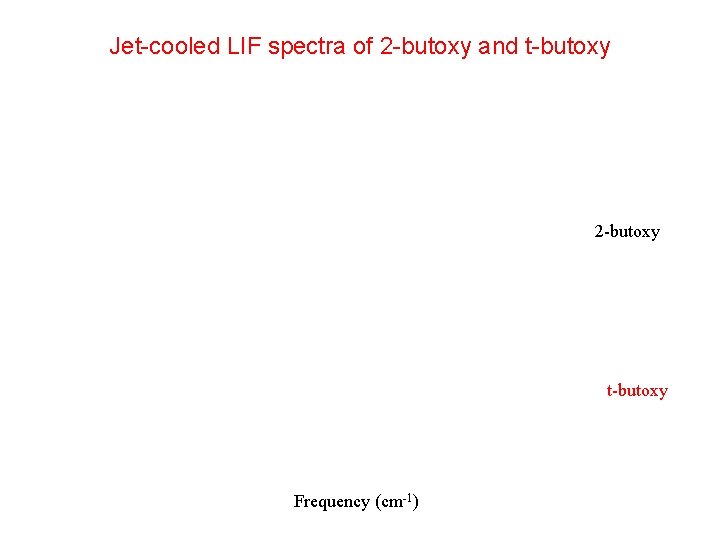
Spectra of Larger Alkoxy Radicals Wang et al. * examined a total of 14 structural isomers of Cn. H 2 n+1 O n=4, 5, 6). For only 4 were structured LIF spectra observed (2 -butoxy, t-butoxy, 3 -pentoxy, t-pentoxy). 2 -Butoxy (213 K) t-Butoxy (213 K) *C. Wang, L. G. Shemesh, W. Deng, M. Lilien, T. S. Dibble, J. Phys. Chem. A 103, 8207 (1999). *C. Wang, W. Dent, L. Shemesh, M. Lilien, D. R. Katz, T. S. Dibble, J. Phys. Chem. A 104, 10368 (2000).

Jet-cooled LIF spectra of 2 -butoxy and t-butoxy 2 -butoxy t-butoxy Frequency (cm-1)

Vibrationally resolved spectrum of 1 -hexoxy

Jet-cooled LIF spectra of various 1 -alkoxies

Jet-cooled LIF spectra of various 2 -alkoxies

Alkoxy Origin Frequencies

Moderate resolution spectra of various 2 -alkoxy origins

Jet-cooled LIF spectra of various 1 -alkoxies

1 -Pentoxy A

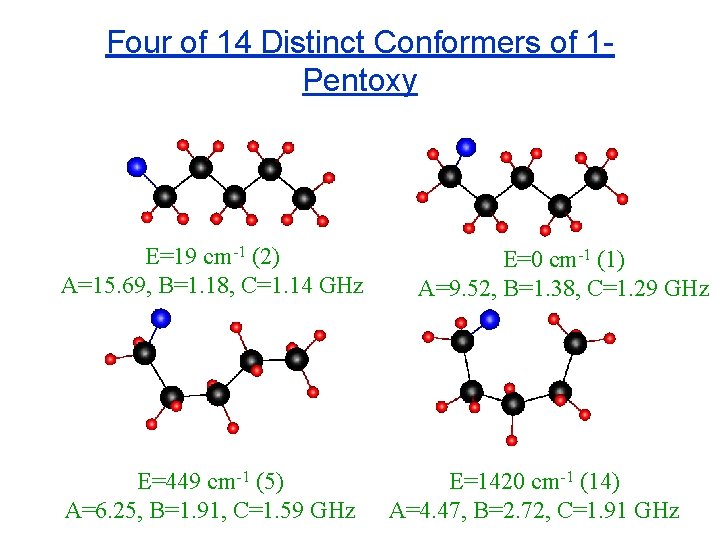
Four of 14 Distinct Conformers of 1 Pentoxy E=19 cm-1 (2) A=15. 69, B=1. 18, C=1. 14 GHz E=449 cm-1 (5) A=6. 25, B=1. 91, C=1. 59 GHz E=0 cm-1 (1) A=9. 52, B=1. 38, C=1. 29 GHz E=1420 cm-1 (14) A=4. 47, B=2. 72, C=1. 91 GHz

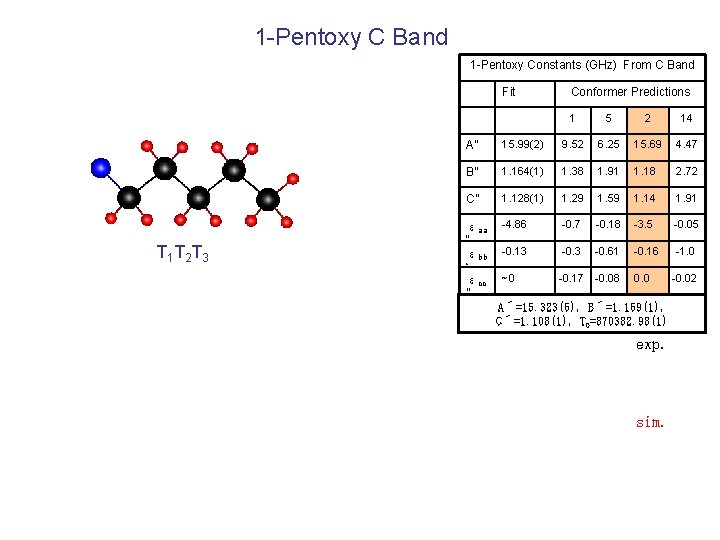
1 -Pentoxy C Band 1 -Pentoxy Constants (GHz) From C Band Fit T 1 T 2 T 3 Conformer Predictions 1 5 2 14 A” 15. 99(2) 9. 52 6. 25 15. 69 4. 47 B” 1. 164(1) 1. 38 1. 91 1. 18 2. 72 C” 1. 128(1) 1. 29 1. 59 1. 14 1. 91 εaa “ εbb -4. 86 -0. 7 -0. 18 -3. 5 -0. 05 -0. 13 -0. 61 -0. 16 -1. 0 ” εcc ” ~0 -0. 17 -0. 08 0. 0 A´=15. 323(6), B´=1. 169(1), C´=1. 108(1), T 0=870382. 98(1) exp. sim. -0. 02

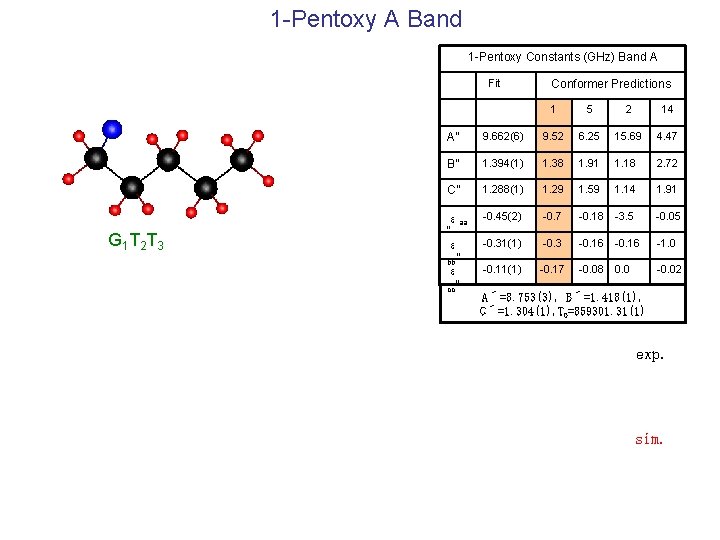
1 -Pentoxy A Band 1 -Pentoxy Constants (GHz) Band A Fit G 1 T 2 T 3 Conformer Predictions 1 5 2 14 A” 9. 662(6) 9. 52 6. 25 15. 69 4. 47 B” 1. 394(1) 1. 38 1. 91 1. 18 2. 72 C” 1. 288(1) 1. 29 1. 59 1. 14 1. 91 εaa “ ε bb” ε cc” -0. 45(2) -0. 7 -0. 18 -3. 5 -0. 05 -0. 31(1) -0. 3 -0. 16 -1. 0 -0. 11(1) -0. 17 -0. 08 0. 0 -0. 02 A´=8. 753(3), B´=1. 418(1), C´=1. 304(1), T 0=859301. 31(1) exp. sim.

Alkoxy LIF Excitation Spectra T Tcco G Tco Gco T 1 T 2 G 1 T 2 T 1 G 2 T 1 T 2 T 3 G 1 T 2 T 3 T 1 T 2 G 3 G 1 T 2 G 3 T 1 T 2 cco T 1 G 2 cco T 1 T 2 T 3 cco G 1 T 2 T 3 co T 1 G 2 T 3 cco T 1 T 2 G 3 co T 1 T 2 T 3 co+cco T 1 T 2 T 3 T 4 G 1 T 2 T 3 T 4 T 1 T 2 T 3 T 4 co G 1 T 2 T 3 T 4 T 5 T 6 T 1 T 2 T 3 T 4 T 5 T 6 G 1 T 2 T 3 T 4 T 5 T 6 T 7 T 1 T 2 T 3 T 4 T 5 T 6 T 7 G 1 T 2 T 3 T 4 T 5 T 6 T 7 T 8 T 1 T 2 T 3 T 4 T 5 co T 1 T 2 T 3 T 4 T 5 T 6 T 7 co T 1 T 2 T 3 T 4 T 5 T 6 T 7 T 8 co

Chemistry and Dynamics • Which Species (Isomers) Observed • Which Conformers Observed ~ • Isomer/Conformer B State Decay

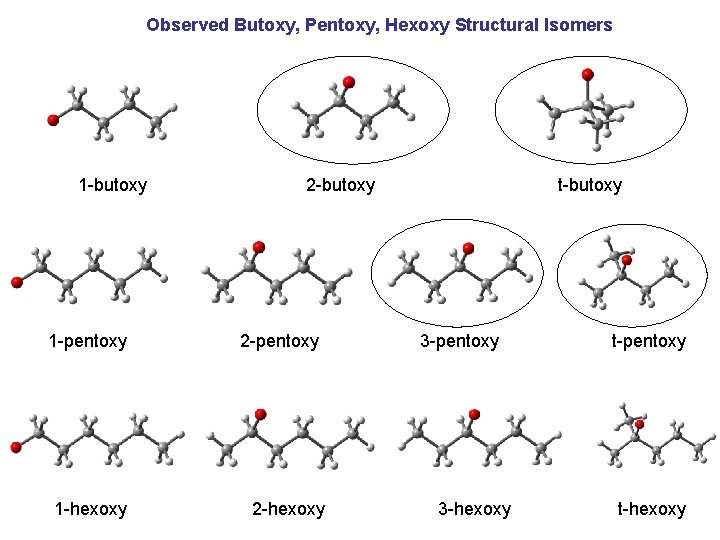
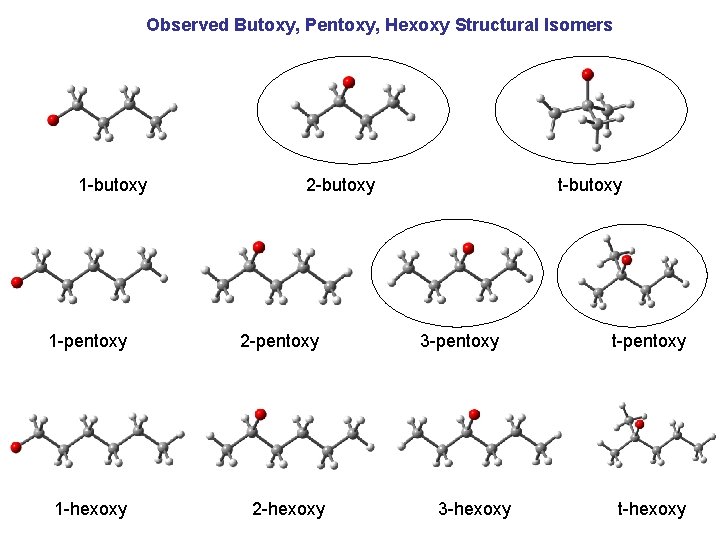
Observed Butoxy, Pentoxy, Hexoxy Structural Isomers 1 -butoxy 1 -pentoxy 1 -hexoxy 2 -butoxy 2 -pentoxy 2 -hexoxy t-butoxy 3 -pentoxy 3 -hexoxy t-pentoxy t-hexoxy

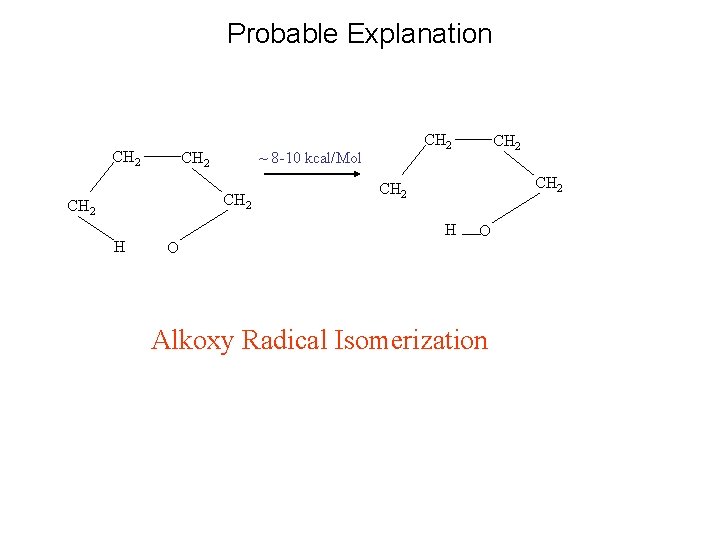
Probable Explanation CH 2 ~ 8 -10 kcal/Mol CH 2 H CH 2 H O CH 2 O Alkoxy Radical Isomerization

Observed Butoxy, Pentoxy, Hexoxy Structural Isomers 1 -butoxy 1 -pentoxy 1 -hexoxy 2 -butoxy 2 -pentoxy 2 -hexoxy t-butoxy 3 -pentoxy 3 -hexoxy t-pentoxy t-hexoxy

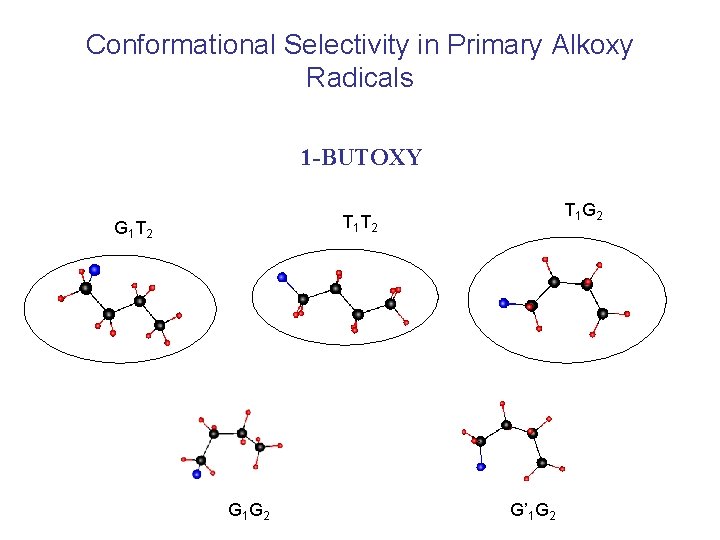
Conformational Selectivity in Primary Alkoxy Radicals 1 -BUTOXY T 1 G 2 T 1 T 2 G 1 G 2 G’ 1 G 2

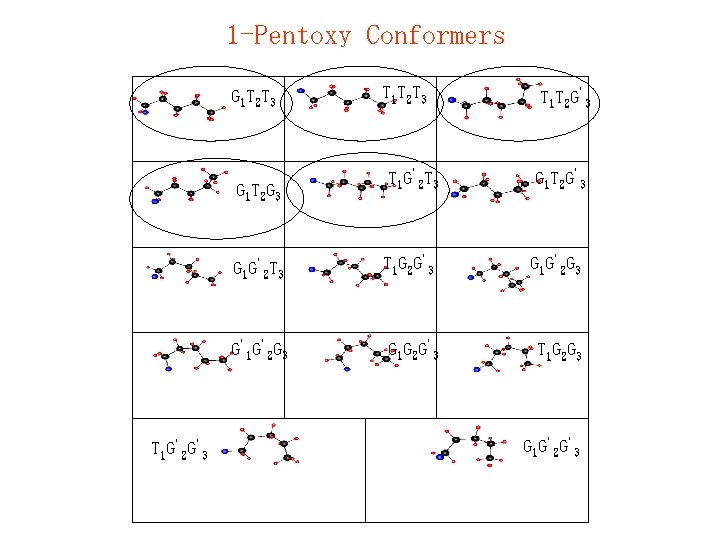
1 -Pentoxy Conformers G 1 T 2 T 3 T 1 T 2 G'3 T 1 G'2 T 3 G 1 T 2 G'3 G 1 G'2 T 3 T 1 G 2 G'3 G 1 G'2 G 3 G'1 G'2 G 3 G 1 G 2 G'3 T 1 G 2 G 3 G 1 T 2 G 3 T 1 G'2 G'3 T 1 T 2 T 3 G 1 G'2 G'3

~ B State Relative Lifetime of Alkoxy Radicals, Cn. H 2 n+1 O, n=1 -3

~ B State Relative Lifetimes of Butoxy Structural Isomers and Conformers

Behavioral Trends of Decay Processes in the Alkoxy Radicals • All radicals exhibit (nearly) pure radiative decay from the vibrationless level with lifetimes in the 1. 0 -2. 5 sec range • Radicals with a single conformer show gradually increasing non-radiative decay when the excess vibrational energy deposited in the CO stretch is varied from 0 to >3000 cm-1 (>5 v. CO) • Radicals with multiple conformers, or equivalently a C-C-C torsion, do not fluorescence with excess energy > 800 cm-1 (~1 v. CO) • All (or nearly all) trans conformers typically exhibit nearly pure radiative decay from v. CO=1 while those involving gauche conformations exhibit almost exclusively non-radiative decay from all levels above the vibrationless

Alkoxy Radical Studies Present • First observation of LIF spectra of nearly 20 large alkoxy radicals, Cn. H 2 n+1 O, n=5 -12. • Detailed spectroscopic analyses determining ground and excited state rotational constants and ground state spin-rotation constants – Primary alkoxies – complete – Secondary, tertiary alkoxies – in progress • Unique correlation of spectral bands and conformers ~ • B State decay and isomer/conformer specific dynamics Future Double resonance experiments to probe ground state vibrational structure and dynamics • Other conformational and isomer specific molecular dynamics

ACKNOWLEDGEMENTS $$$ U. S. National Science Foundation and Department of Energy $$$ FB 08, 59 th MSS; MF 04, 06 58 th MSS; TD 04, 57 th MSS; TD 05 57 th MSS; WH 06, 56 th MSS; WE 04, 55 th MSS Graduate Students MF 05, 58 th MSS; TA 10, 56 th MSS Post-docs Sandhya Gopalakrishnan – KLA-Tencor Lily Zu – Beijing Normal University György Tarczay – Vadim Stakhursky – OSU Ph. D, June, 2005 Eötvös University RJ 03, 59 th MSS; RG 08, 58 th MSS; RF 03, 57 th MSS MH 10, TJ 05 60 th MSS; WJ 08, 59 th MSS; TC 06 56 th MSS Current Group Members Jinjun Liu - OSU MH 09, 60 th MSS; TI 12 59 th MSS; RG 09, 58 th MSS Sergey Zalyubovsky – GE Global Research Center TI 08, 59 th MSS; TG 04, 58 th MSS; WJ 05, 57 th MSS; WH 11, 12, 56 th MSS RE 05 TJ 04 MH 15 RJ 01 Gabriel Just TJ 11 John Yi Erin Sharp Ilias Sioutis Shenhai Wu TJ 10 Patrick Dupré

Alkoxy Potential Curves

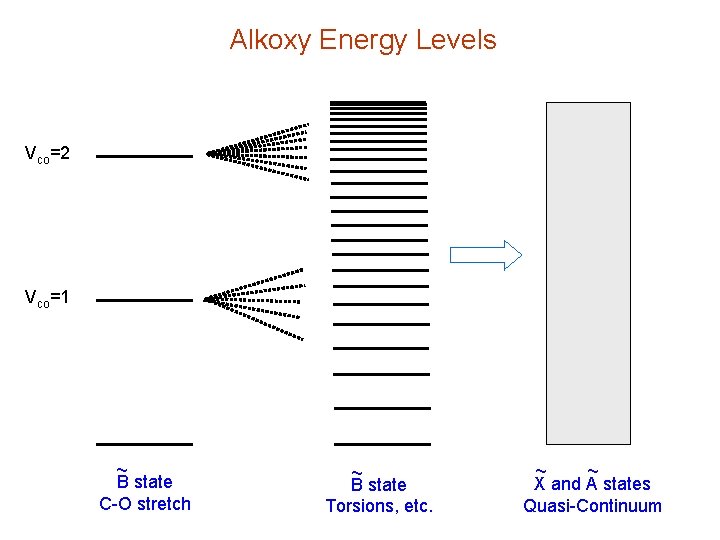
Alkoxy Energy Levels Vco=2 Vco=1 ~ B state C-O stretch ~ B state Torsions, etc. ~ ~ X and A states Quasi-Continuum

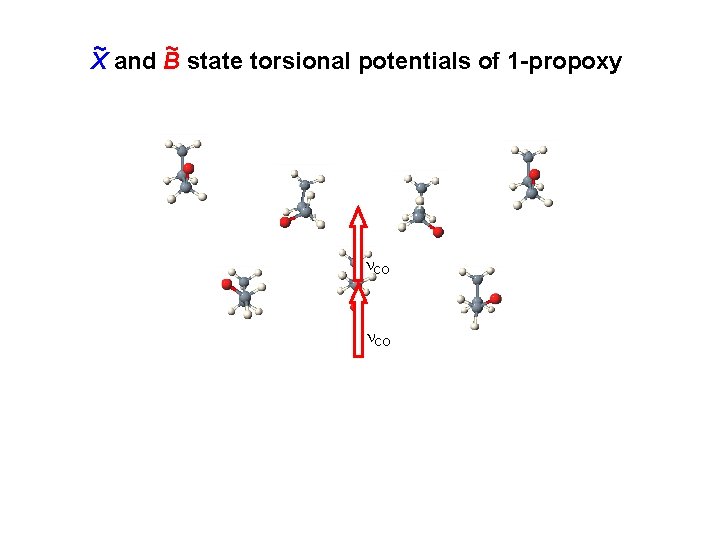
~ and B ~ state torsional potentials of 1 -propoxy X CO

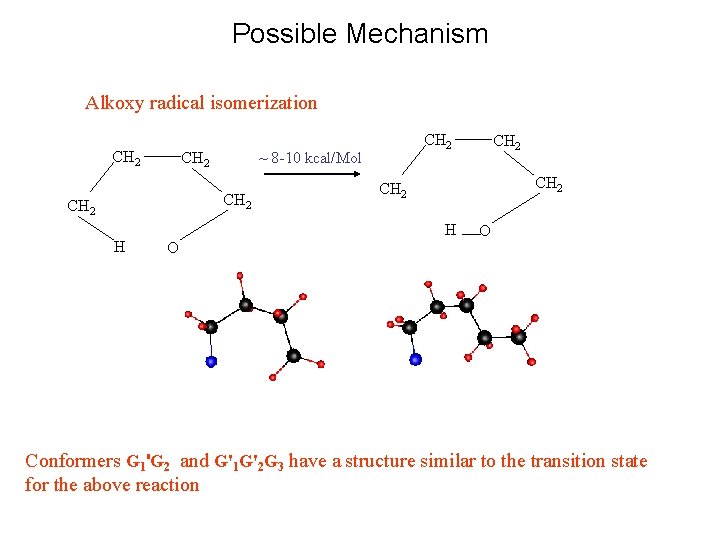
Possible Mechanism Alkoxy radical isomerization CH 2 ~ 8 -10 kcal/Mol CH 2 H CH 2 H O CH 2 O Conformers G 1'G 2 and G'1 G'2 G 3 have a structure similar to the transition state for the above reaction

Alkoxy LIF Excitation Spectra T Tcco G Tco Gco T 1 T 2 G 1 T 2 T 1 G 2 T 1 T 2 T 3 G 1 T 2 T 3 T 1 T 2 G 3 G 1 T 2 G 3 T 1 T 2 cco T 1 G 2 cco T 1 T 2 T 3 cco G 1 T 2 T 3 co T 1 G 2 T 3 cco T 1 T 2 G 3 co T 1 T 2 T 3 co+cco T 1 T 2 T 3 T 4 G 1 T 2 T 3 T 4 T 1 T 2 T 3 T 4 co G 1 T 2 T 3 T 4 T 5 T 6 T 1 T 2 T 3 T 4 T 5 T 6 G 1 T 2 T 3 T 4 T 5 T 6 T 7 T 1 T 2 T 3 T 4 T 5 T 6 T 7 G 1 T 2 T 3 T 4 T 5 T 6 T 7 T 8 T 1 T 2 T 3 T 4 T 5 co T 1 T 2 T 3 T 4 T 5 T 6 T 7 co T 1 T 2 T 3 T 4 T 5 T 6 T 7 T 8 co