Hierarchy of Decisions One Level1 Decision Batch vs

Hierarchy of Decisions

One Level-1 Decision: Batch vs. Continuous


Hierarchy of Decisions

Heuristics: Recover more than 99% of all valuable materials. Therefore, it is reasonable to assume Completely recover and recycle all valuable reactants
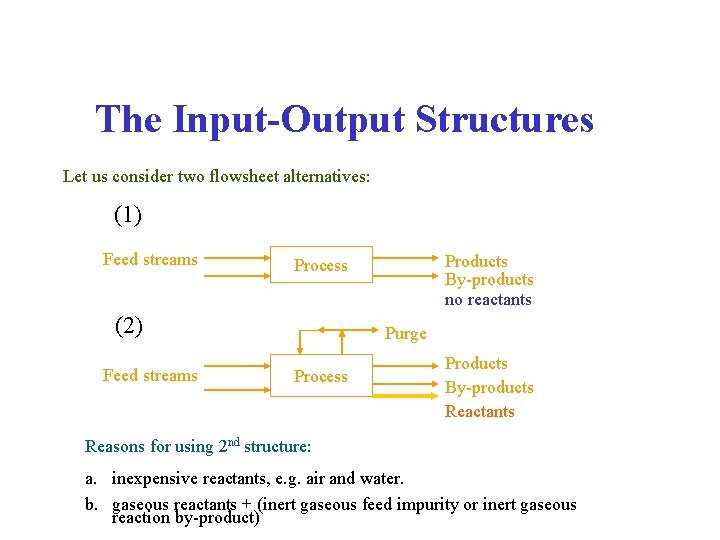
The Input-Output Structures Let us consider two flowsheet alternatives: (1) Feed streams (2) Feed streams Products By-products no reactants Process Purge Process Products By-products Reactants Reasons for using 2 nd structure: a. inexpensive reactants, e. g. air and water. b. gaseous reactants + (inert gaseous feed impurity or inert gaseous reaction by-product)
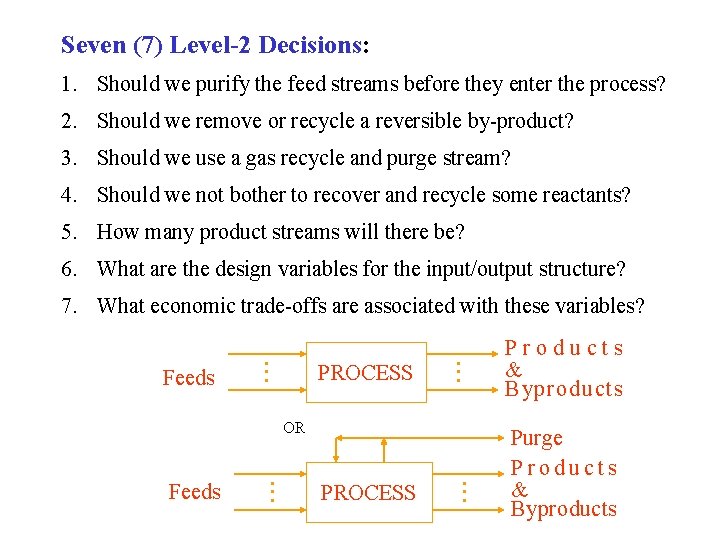
Seven (7) Level-2 Decisions: 1. Should we purify the feed streams before they enter the process? 2. Should we remove or recycle a reversible by-product? 3. Should we use a gas recycle and purge stream? 4. Should we not bother to recover and recycle some reactants? 5. How many product streams will there be? 6. What are the design variables for the input/output structure? 7. What economic trade-offs are associated with these variables? Feeds PROCESS Products & Byproducts OR Feeds PROCESS Purge Products & Byproducts
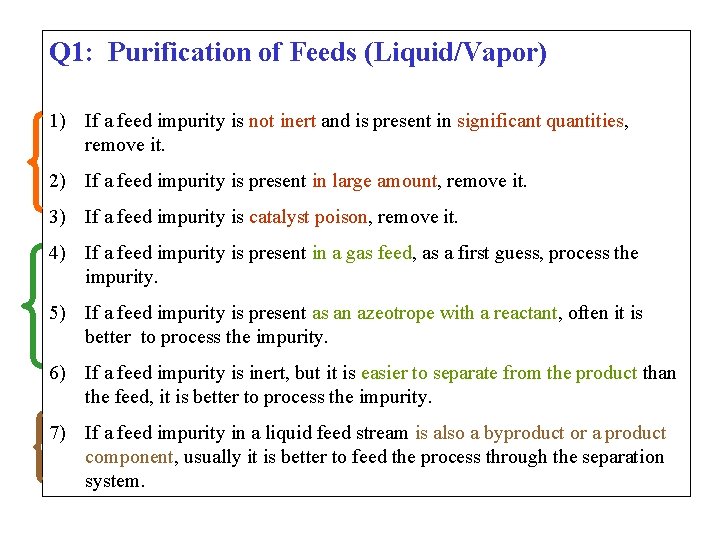
Q 1: Purification of Feeds (Liquid/Vapor) 1) If a feed impurity is not inert and is present in significant quantities, remove it. 2) If a feed impurity is present in large amount, remove it. 3) If a feed impurity is catalyst poison, remove it. 4) If a feed impurity is present in a gas feed, as a first guess, process the impurity. 5) If a feed impurity is present as an azeotrope with a reactant, often it is better to process the impurity. 6) If a feed impurity is inert, but it is easier to separate from the product than the feed, it is better to process the impurity. 7) If a feed impurity in a liquid feed stream is also a byproduct or a product component, usually it is better to feed the process through the separation system.
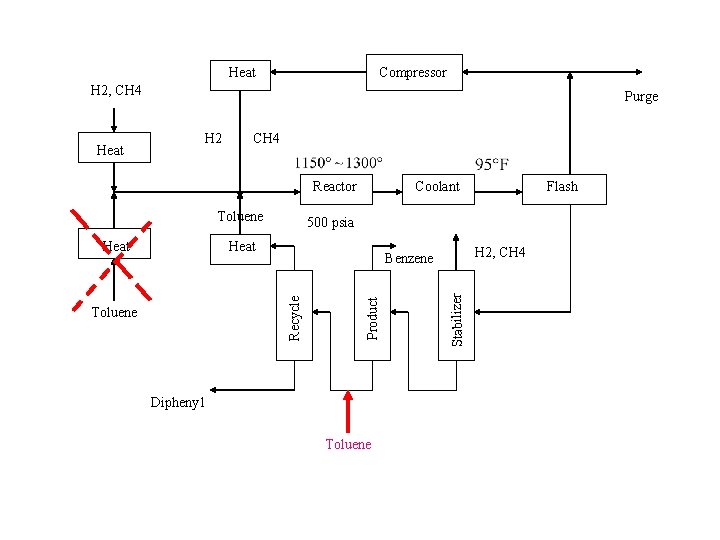
Heat Compressor H 2, CH 4 H 2 CH 4 Reactor Toluene Heat H 2, CH 4 Benzene Product Toluene Flash 500 psia Recycle Heat Coolant Dipheny 1 Toluene Stabilizer Heat Purge

Q 3: Gas Recycle and Purge 1. “Light” reactant + “Light” feed impurity 2. “Light” reactant + “Light” by-product produced by a reaction ü Whenever a light reactant and either a light feed impurity or a light byproduct boil lower than propylene (-55ºF), use a gas recycle and purge stream. ü Lower boiling components normally cannot be condensed at high pressure with cooling water.
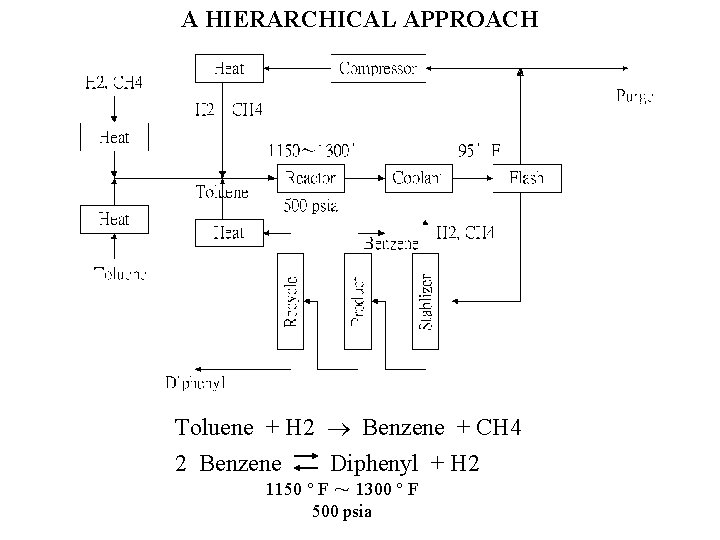
A HIERARCHICAL APPROACH Toluene + H 2 Benzene + CH 4 2 Benzene Diphenyl + H 2 1150 F ~ 1300 F 500 psia

Q 4: Do not recover and recycle some reactants which are inexpensive, e. g. , air and water. We could try to make them react completely, but often we feed them as an excess to try to force some more valuable reactants to completion.
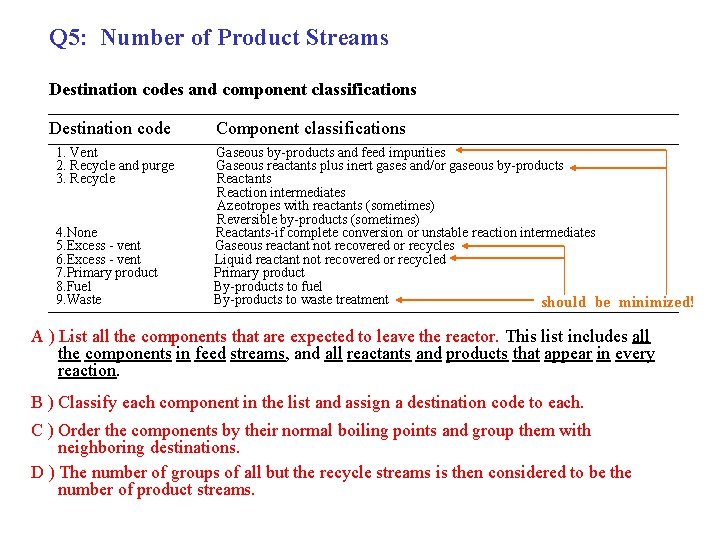
Q 5: Number of Product Streams Destination codes and component classifications Destination code 1. Vent 2. Recycle and purge 3. Recycle 4. None 5. Excess - vent 6. Excess - vent 7. Primary product 8. Fuel 9. Waste Component classifications Gaseous by-products and feed impurities Gaseous reactants plus inert gases and/or gaseous by-products Reactants Reaction intermediates Azeotropes with reactants (sometimes) Reversible by-products (sometimes) Reactants-if complete conversion or unstable reaction intermediates Gaseous reactant not recovered or recycles Liquid reactant not recovered or recycled Primary product By-products to fuel By-products to waste treatment should be minimized! A ) List all the components that are expected to leave the reactor. This list includes all the components in feed streams, and all reactants and products that appear in every reaction. B ) Classify each component in the list and assign a destination code to each. C ) Order the components by their normal boiling points and group them with neighboring destinations. D ) The number of groups of all but the recycle streams is then considered to be the number of product streams.
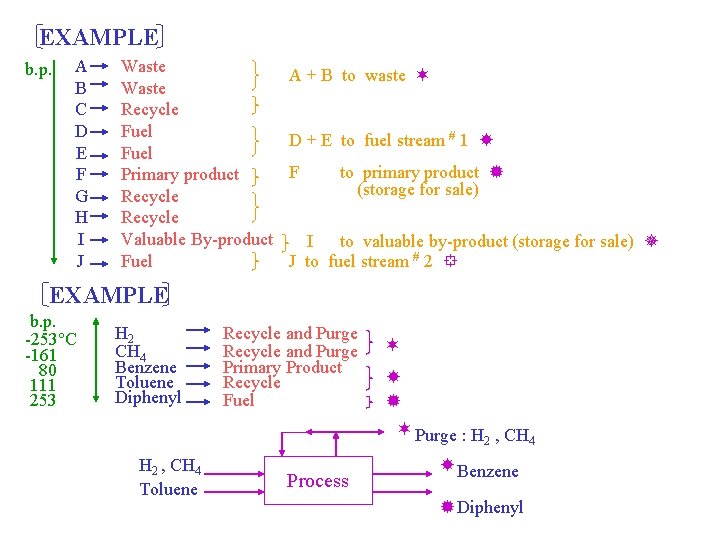
EXAMPLE b. p. A B C D E F G H I J Waste Recycle Fuel Primary product Recycle Valuable By-product Fuel A + B to waste D + E to fuel stream # 1 F to primary product (storage for sale) I to valuable by-product (storage for sale) J to fuel stream # 2 EXAMPLE b. p. -253 C -161 80 111 253 H 2 CH 4 Benzene Toluene Diphenyl Recycle and Purge Primary Product Recycle Fuel Purge : H , CH 2 4 H 2 , CH 4 Toluene Process Benzene Diphenyl
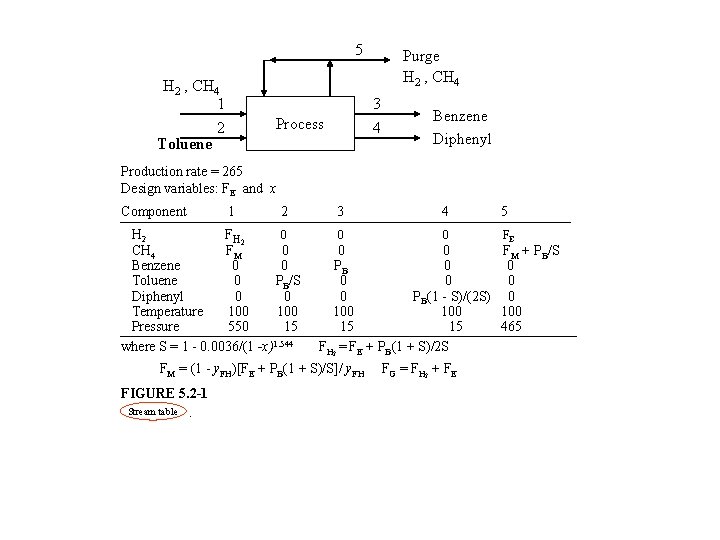
5 H 2 , CH 4 1 2 Toluene Purge H 2 , CH 4 3 4 Process Benzene Diphenyl Production rate = 265 Design variables: FE and x Component 1 2 H 2 F H 2 0 CH 4 FM 0 Benzene 0 0 Toluene 0 PB/S Diphenyl 0 0 Temperature 100 Pressure 550 15 where S = 1 - 0. 0036/(1 -x)1. 544 3 4 5 0 0 FE 0 0 FM + PB/S PB 0 0 0 PB(1 - S)/(2 S) 0 100 100 15 15 465 FH 2 = FE + PB(1 + S)/2 S FM = (1 - y. FH)[FE + PB(1 + S)/S]/ y. FH FIGURE 5. 2 -1 Stream table . FG = FH 2 + FE

Alternatives for the HDA Process 1. Purify the H 2 feed stream. 2. Recycle diphenyl 3. Purify H 2 recycle stream.
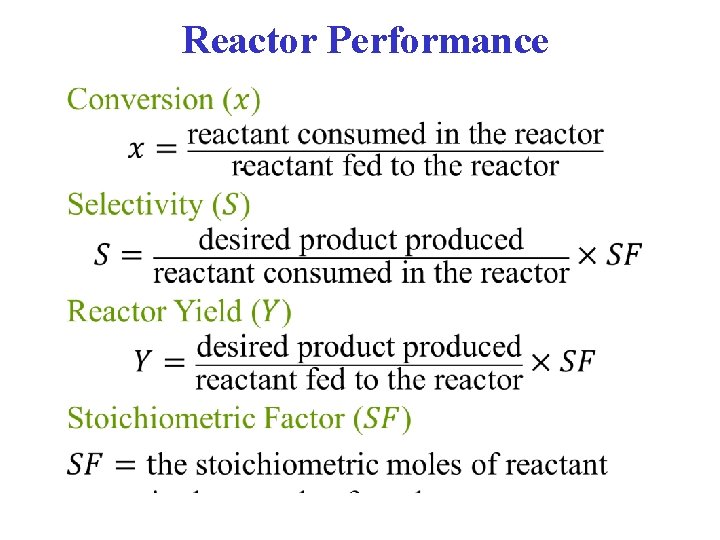
Reactor Performance •
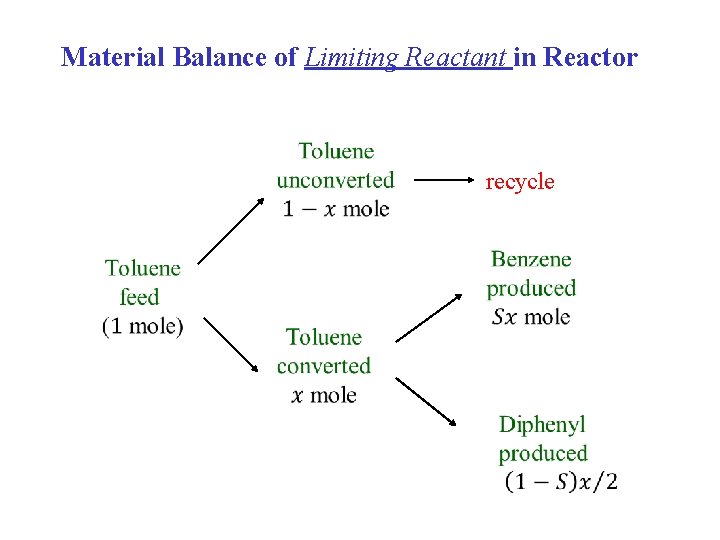
Material Balance of Limiting Reactant in Reactor recycle
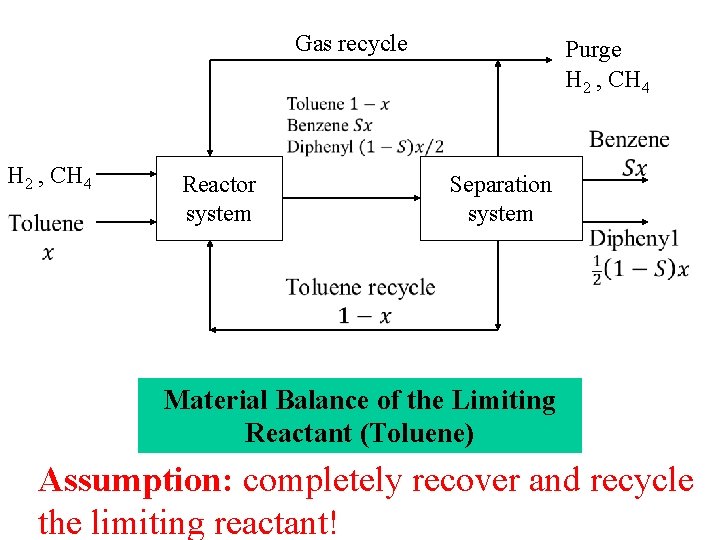
Gas recycle H 2 , CH 4 Reactor system Purge H 2 , CH 4 Separation system Material Balance of the Limiting Reactant (Toluene) Assumption: completely recover and recycle the limiting reactant!
Q 6: Design Variables
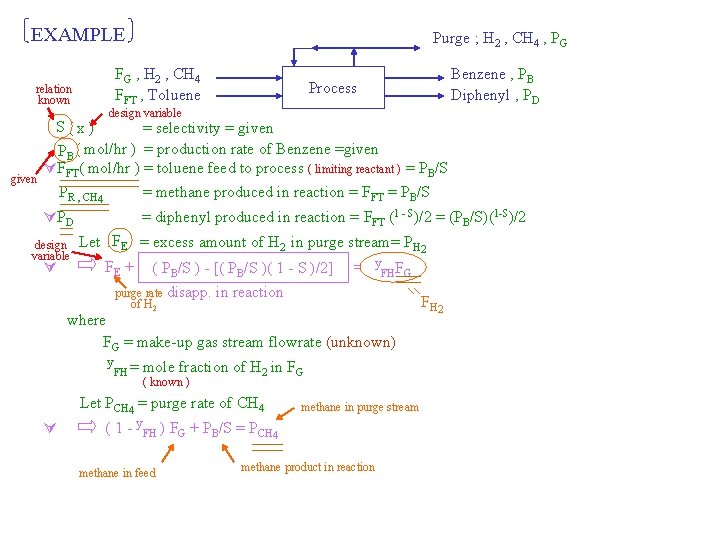
EXAMPLE relation known given Purge ; H 2 , CH 4 , PG FG , H 2 , CH 4 FFT , Toluene Benzene , PB Diphenyl , PD Process design variable SS( x ) = selectivity = given PBB( mol/hr ) = production rate of Benzene =given FFT( mol/hr ) = toluene feed to process ( limiting reactant ) = PB/S PR , CH 4 = methane produced in reaction = FFT = PB/S PD design variable = diphenyl produced in reaction = FFT (1 - S)/2 = (PB/S)(1 -S)/2 Let FFEE = excess amount of H 2 in purge stream= PH 2 FE + ( PB/S ) - [( PB/S )( 1 - S )/2] purge rate disapp. of H 2 y FF = y. FH FH GG in reaction where FG = make-up gas stream flowrate (unknown) y FH = mole fraction of H 2 in FG ( known ) Let PCH 4 = purge rate of CH 4 methane in purge stream ( 1 - y. FH ) FG + PB/S = PCH 4 methane in feed methane product in reaction F H 2
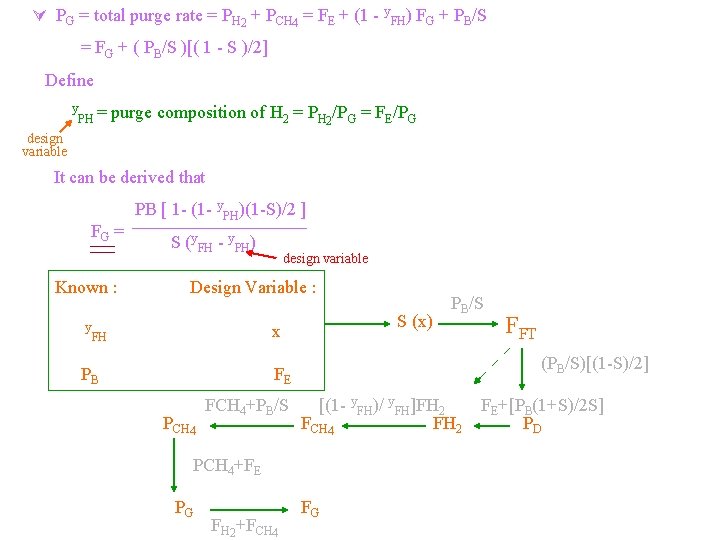
PG = total purge rate = PH 2 + PCH 4 = FE + (1 - y. FH) FG + PB/S = FG + ( PB/S )[( 1 - S )/2] Define y PH = purge composition of H 2 = PH 2/PG = FE/PG design variable It can be derived that FG = Known : y PB [ 1 - (1 - y. PH)(1 -S)/2 ] S (y. FH - y. PH) design variable Design Variable : S (x) x FH PB PB/S (PB/S)[(1 -S)/2] FE PCH 4 FCH 4+PB/S [(1 - y. FH)/ y. FH]FH 2 FCH 4 FH 2 PCH 4+FE PG FH 2+FCH 4 FFT FG FE+[PB(1+S)/2 S] PD
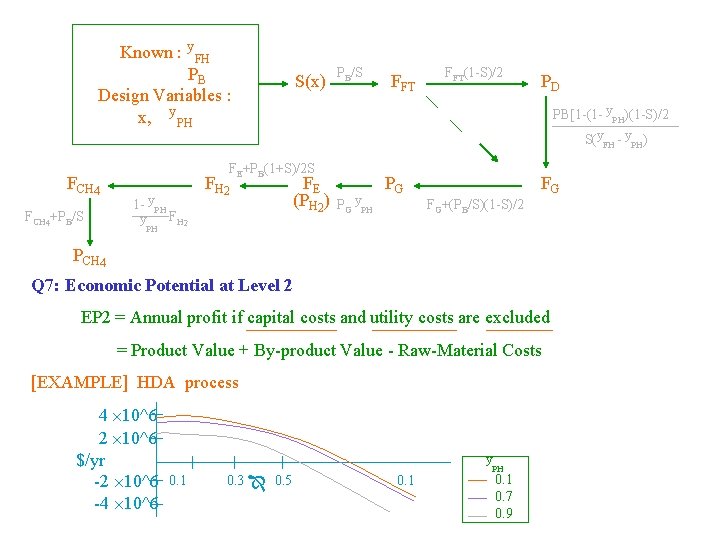
Known : y. FH PB Design Variables : x, y. PH FCH 4+PB/S S(x) PB/S y FH 2 PH PH FH 2 FFT(1 -S)/2 PD PB[1 -(1 - y. PH)(1 -S)/2 S(y. FH - y. PH) FE+PB(1+S)/2 S 1 - y FFT FE (PH 2) PG y. PH PG FG FG+(PB/S)(1 -S)/2 PCH 4 Q 7: Economic Potential at Level 2 EP 2 = Annual profit if capital costs and utility costs are excluded = Product Value + By-product Value - Raw-Material Costs [EXAMPLE] HDA process 4 10^6 2 10^6 $/yr -2 10^6 -4 10^6 y 0. 1 0. 3 0. 5 0. 1 PH 0. 1 0. 7 0. 9

End of Level 2

Douglas, J. M. , “Process Synthesis for Waste Minimization. ” Ind. Eng. Chem. Res. , 1992, 31, 238 -243 If we produce waste by-products, then we have negative byproduct values. Solid waste : land fill cost / lb Contaminated waste water : - sewer charge : $ / 1000 gal. (e. g. $0. 2 / 1000 gal) - waste treatment charge : $ / lb BOD / lb organic compound (e. g. $0. 25 /lb BOD) Solid or liquid waste to be incinerated : $ 0. 65 / lb BOD - biological oxygen demand
- Slides: 28