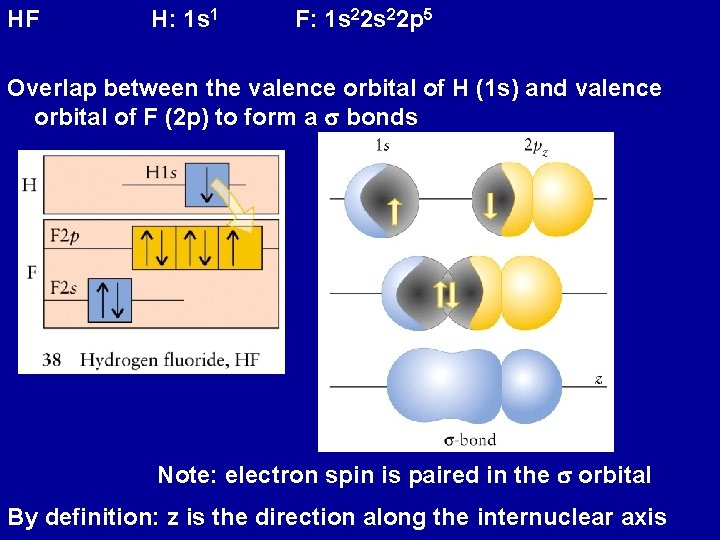
HF H 1 s 1 F 1 s

![PCl 5 P: [Ne] 3 s 2 3 p 3 P 3 p Cl: PCl 5 P: [Ne] 3 s 2 3 p 3 P 3 p Cl:](https://slidetodoc.com/presentation_image/6c0230dff2aa31b51289778f4721f293/image-16.jpg)
![SF 6 S: [Ne] 3 s 2 3 p 4 F: [He] 2 s SF 6 S: [Ne] 3 s 2 3 p 4 F: [He] 2 s](https://slidetodoc.com/presentation_image/6c0230dff2aa31b51289778f4721f293/image-19.jpg)
- Slides: 26

HF H: 1 s 1 F: 1 s 22 p 5 Overlap between the valence orbital of H (1 s) and valence orbital of F (2 p) to form a s bonds Note: electron spin is paired in the s orbital By definition: z is the direction along the internuclear axis

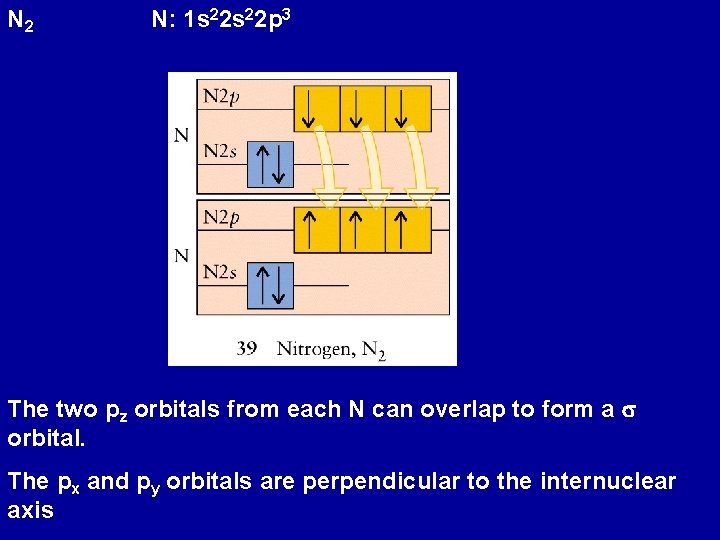
N 2 N: 1 s 22 p 3 The two pz orbitals from each N can overlap to form a s orbital. The px and py orbitals are perpendicular to the internuclear axis

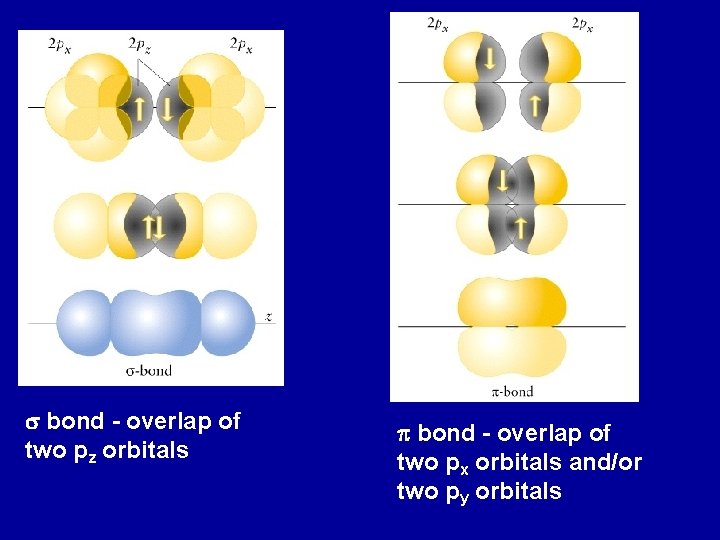
s bond - overlap of two pz orbitals p bond - overlap of two px orbitals and/or two py orbitals

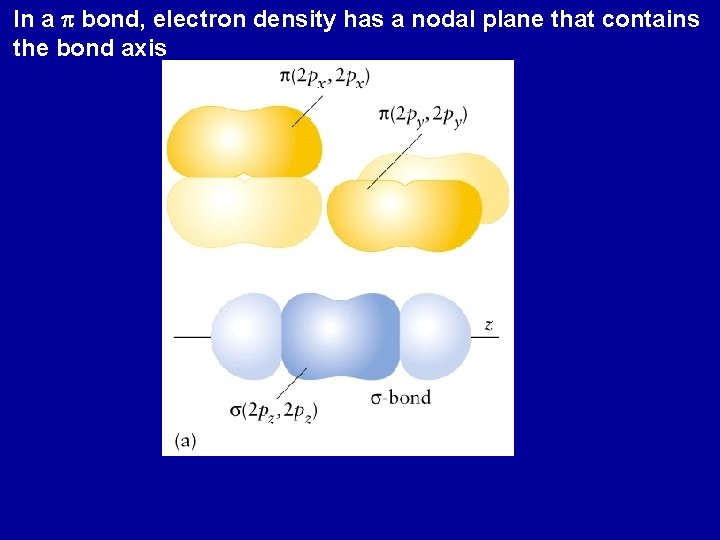
In a p bond, electron density has a nodal plane that contains the bond axis

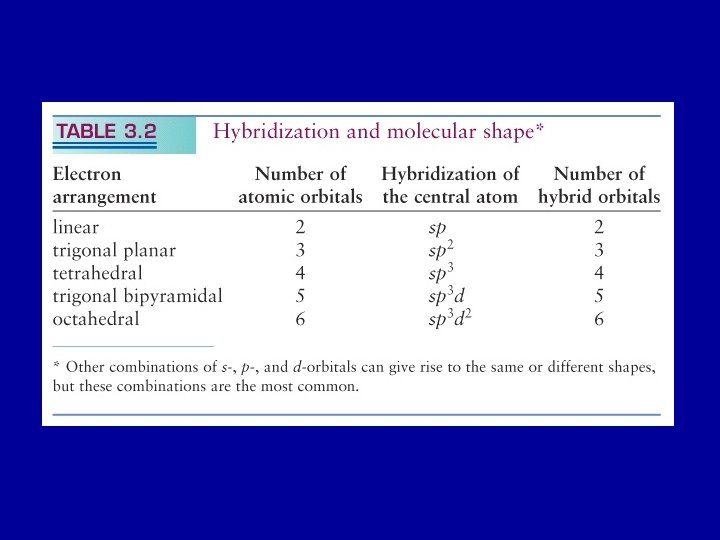
According to the VB theory A single bond is a s-bond A double bond is s-bond plus a p-bond A triple bond is a s-bond and two p-bonds. VB theory: assumes bonds form when unpaired electrons in valence shell atomic orbitals pair the atomic orbitals overlap end to form s-bonds or side by side to form p-bonds.

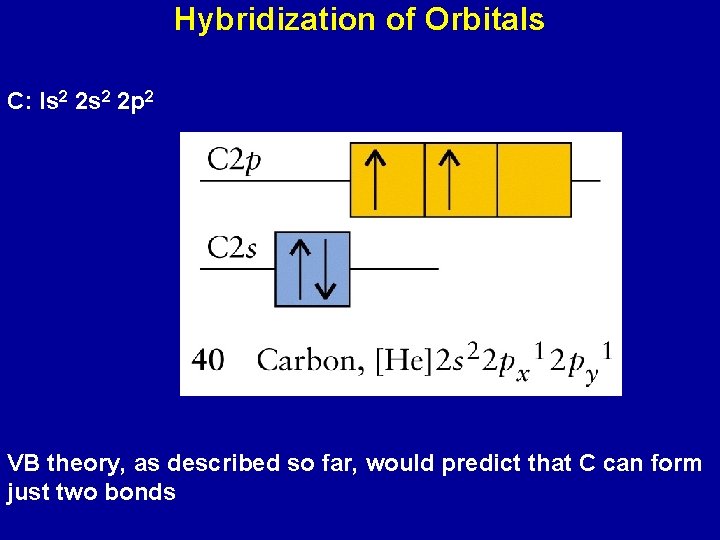
Hybridization of Orbitals C: Is 2 2 p 2 VB theory, as described so far, would predict that C can form just two bonds

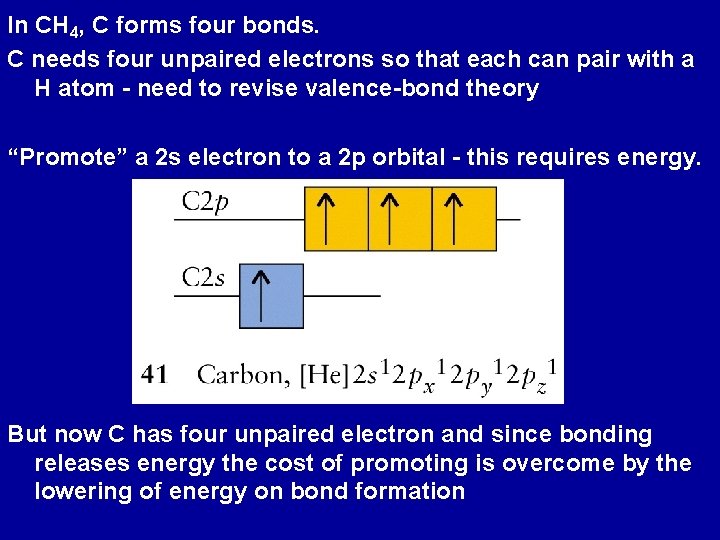
In CH 4, C forms four bonds. C needs four unpaired electrons so that each can pair with a H atom - need to revise valence-bond theory “Promote” a 2 s electron to a 2 p orbital - this requires energy. But now C has four unpaired electron and since bonding releases energy the cost of promoting is overcome by the lowering of energy on bond formation

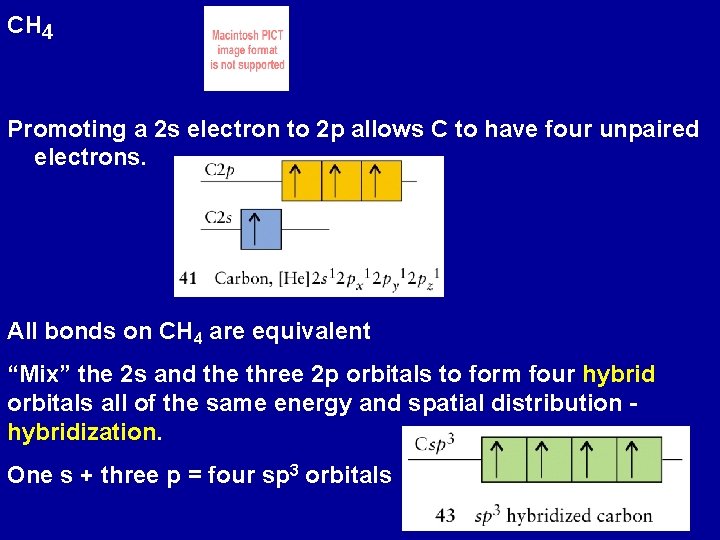
CH 4 Promoting a 2 s electron to 2 p allows C to have four unpaired electrons. All bonds on CH 4 are equivalent “Mix” the 2 s and the three 2 p orbitals to form four hybrid orbitals all of the same energy and spatial distribution hybridization. One s + three p = four sp 3 orbitals


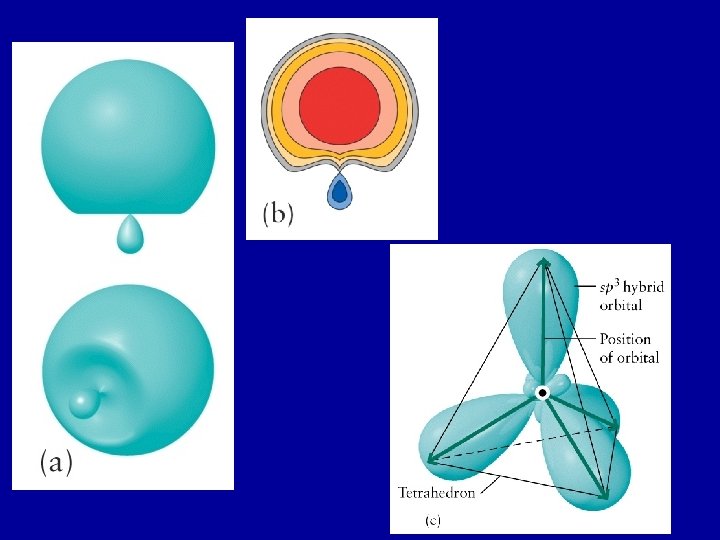
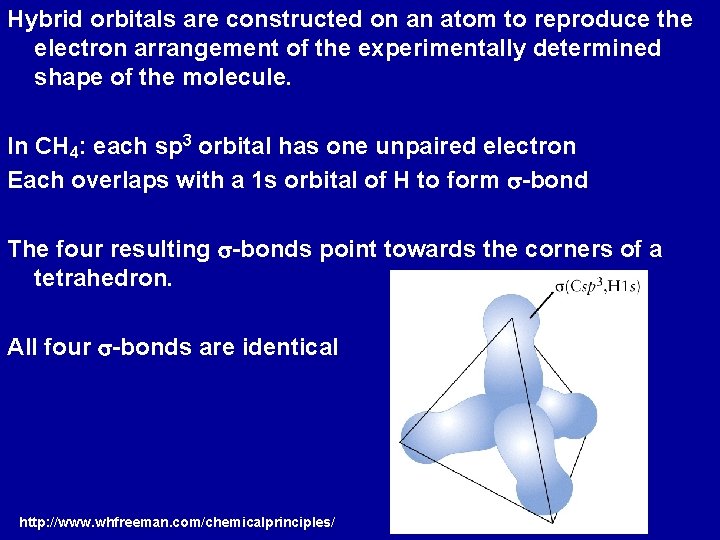
Hybrid orbitals are constructed on an atom to reproduce the electron arrangement of the experimentally determined shape of the molecule. In CH 4: each sp 3 orbital has one unpaired electron Each overlaps with a 1 s orbital of H to form s-bond The four resulting s-bonds point towards the corners of a tetrahedron. All four s-bonds are identical http: //www. whfreeman. com/chemicalprinciples/

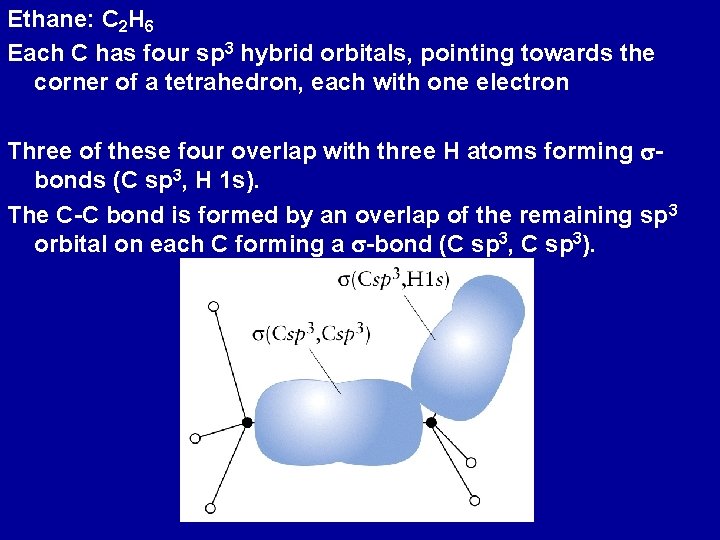
Ethane: C 2 H 6 Each C has four sp 3 hybrid orbitals, pointing towards the corner of a tetrahedron, each with one electron Three of these four overlap with three H atoms forming sbonds (C sp 3, H 1 s). The C-C bond is formed by an overlap of the remaining sp 3 orbital on each C forming a s-bond (C sp 3, C sp 3).

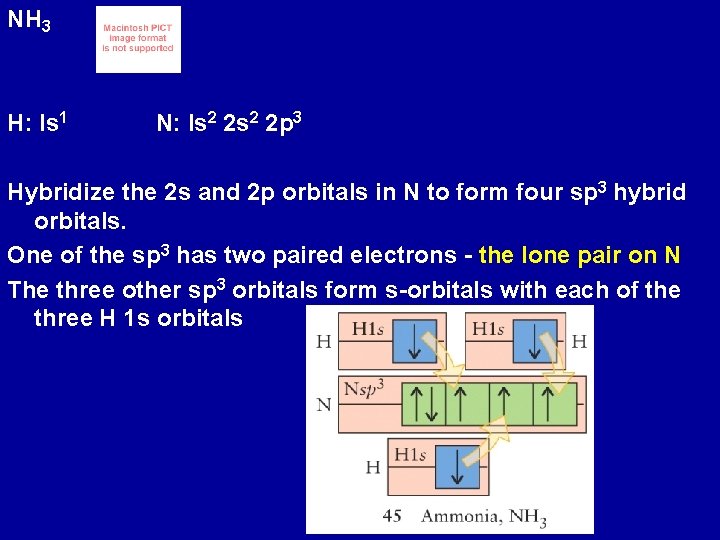
NH 3 H: Is 1 N: Is 2 2 p 3 Hybridize the 2 s and 2 p orbitals in N to form four sp 3 hybrid orbitals. One of the sp 3 has two paired electrons - the lone pair on N The three other sp 3 orbitals form s-orbitals with each of the three H 1 s orbitals

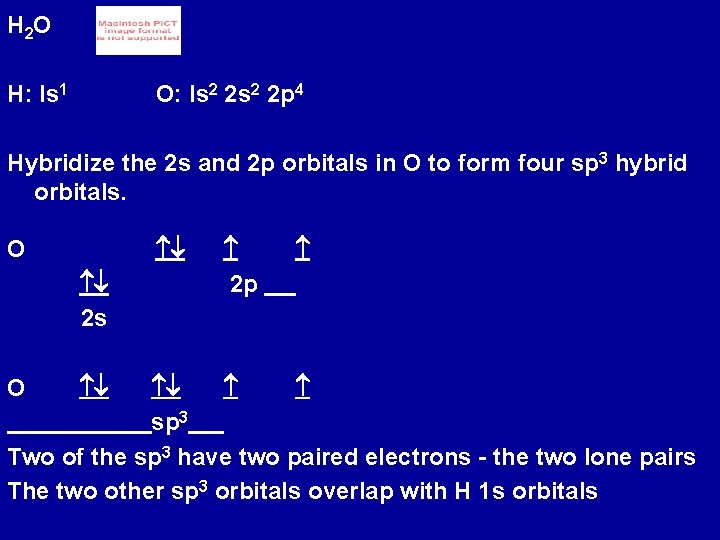
H 2 O H: Is 1 O: Is 2 2 p 4 Hybridize the 2 s and 2 p orbitals in O to form four sp 3 hybrid orbitals. O 2 s sp 3 Two of the sp 3 have two paired electrons - the two lone pairs The two other sp 3 orbitals overlap with H 1 s orbitals O 2 p

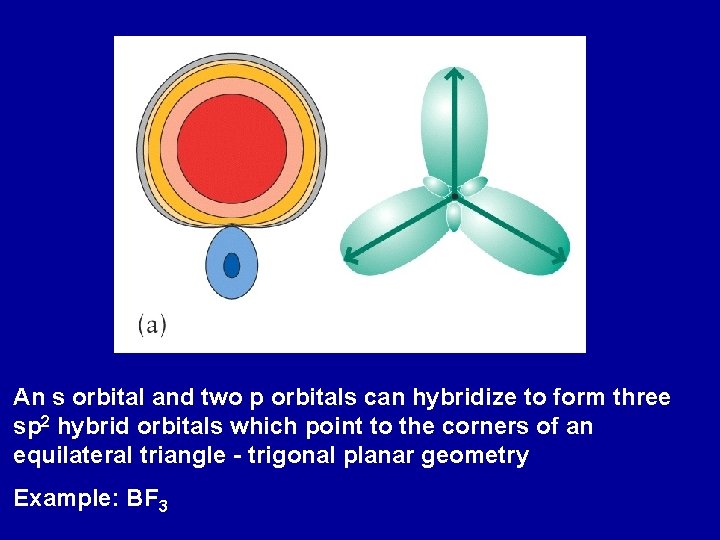
An s orbital and two p orbitals can hybridize to form three sp 2 hybrid orbitals which point to the corners of an equilateral triangle - trigonal planar geometry Example: BF 3

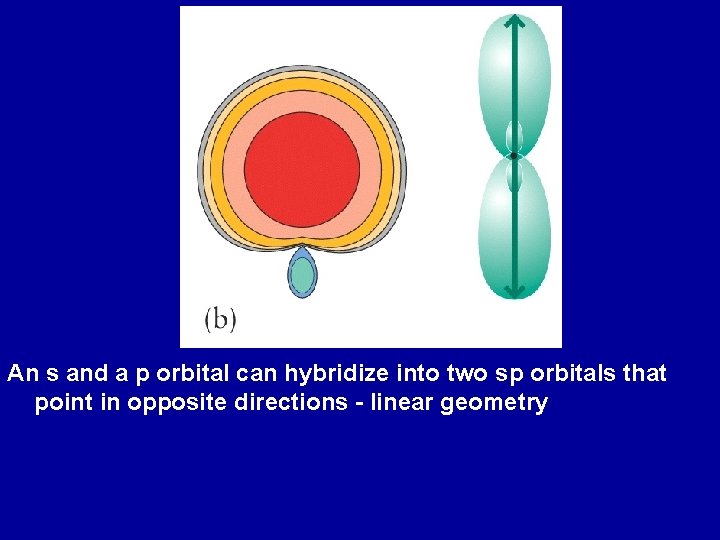
An s and a p orbital can hybridize into two sp orbitals that point in opposite directions - linear geometry
![PCl 5 P Ne 3 s 2 3 p 3 P 3 p Cl PCl 5 P: [Ne] 3 s 2 3 p 3 P 3 p Cl:](https://slidetodoc.com/presentation_image/6c0230dff2aa31b51289778f4721f293/image-16.jpg)
PCl 5 P: [Ne] 3 s 2 3 p 3 P 3 p Cl: [Ne] 3 s 2 3 p 5 Cl 3 s 3 p _ _ 3 s Promote a 3 s electron to the 3 d orbital P sp 3 d _ _ empty 3 d Valence shell expansion - expansion to include d orbitals along with s and p orbitals

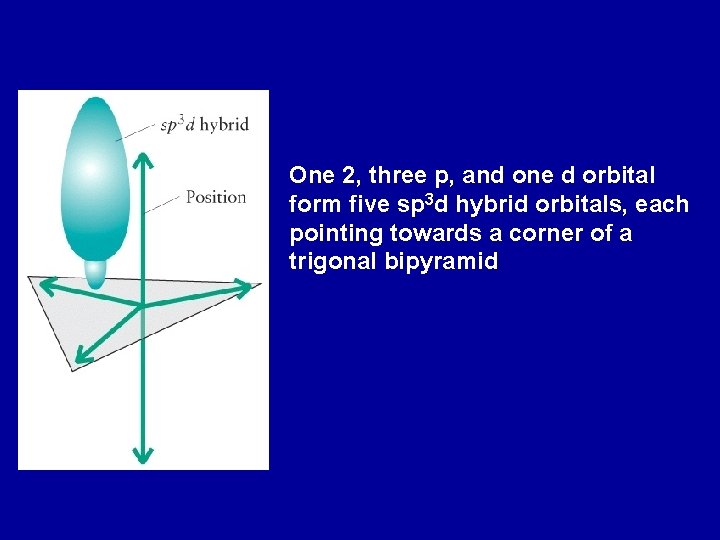
One 2, three p, and one d orbital form five sp 3 d hybrid orbitals, each pointing towards a corner of a trigonal bipyramid

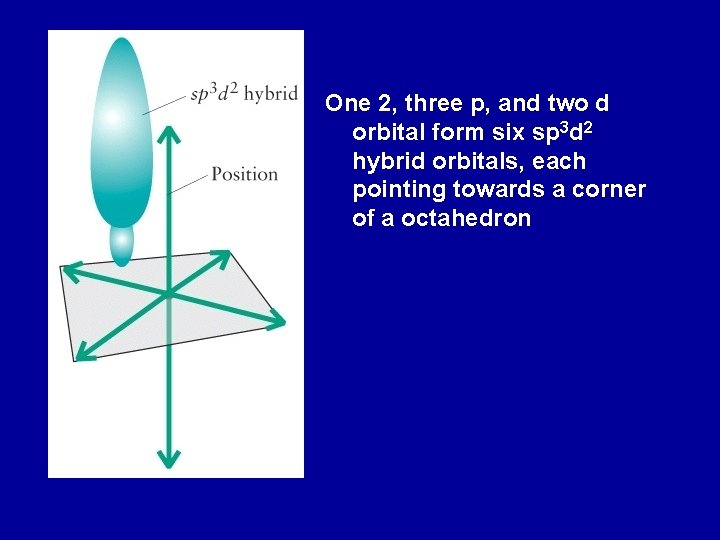
One 2, three p, and two d orbital form six sp 3 d 2 hybrid orbitals, each pointing towards a corner of a octahedron
![SF 6 S Ne 3 s 2 3 p 4 F He 2 s SF 6 S: [Ne] 3 s 2 3 p 4 F: [He] 2 s](https://slidetodoc.com/presentation_image/6c0230dff2aa31b51289778f4721f293/image-19.jpg)
SF 6 S: [Ne] 3 s 2 3 p 4 F: [He] 2 s 2 2 p 5 S 3 p 3 s Include two 3 d orbital and hybridize one s, three p and two d S sp 3 d 2 _ _ _ empty 3 d


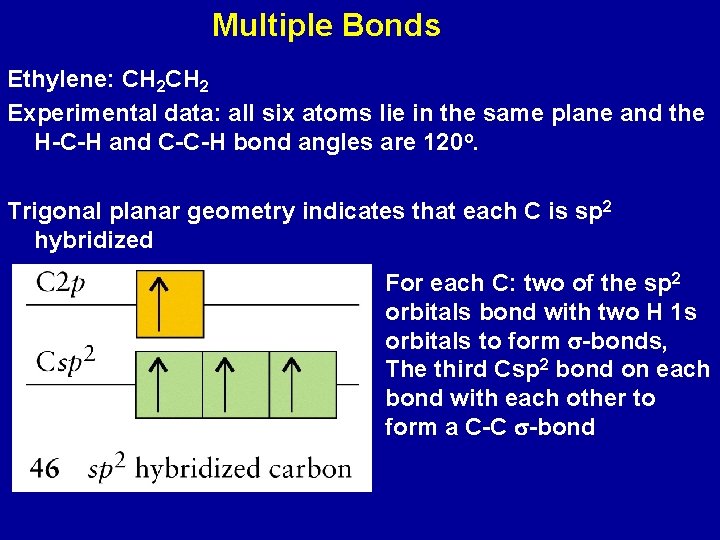
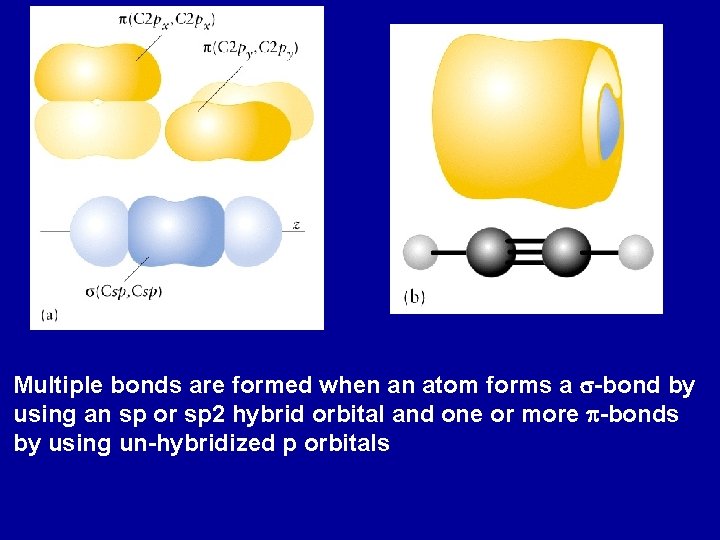
Multiple Bonds Ethylene: CH 2 Experimental data: all six atoms lie in the same plane and the H-C-H and C-C-H bond angles are 120 o. Trigonal planar geometry indicates that each C is sp 2 hybridized For each C: two of the sp 2 orbitals bond with two H 1 s orbitals to form s-bonds, The third Csp 2 bond on each bond with each other to form a C-C s-bond

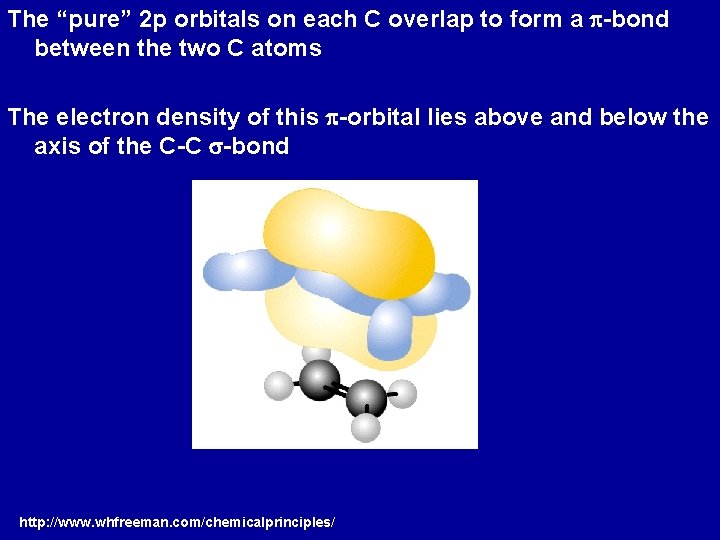
The “pure” 2 p orbitals on each C overlap to form a p-bond between the two C atoms The electron density of this p-orbital lies above and below the axis of the C-C s-bond http: //www. whfreeman. com/chemicalprinciples/

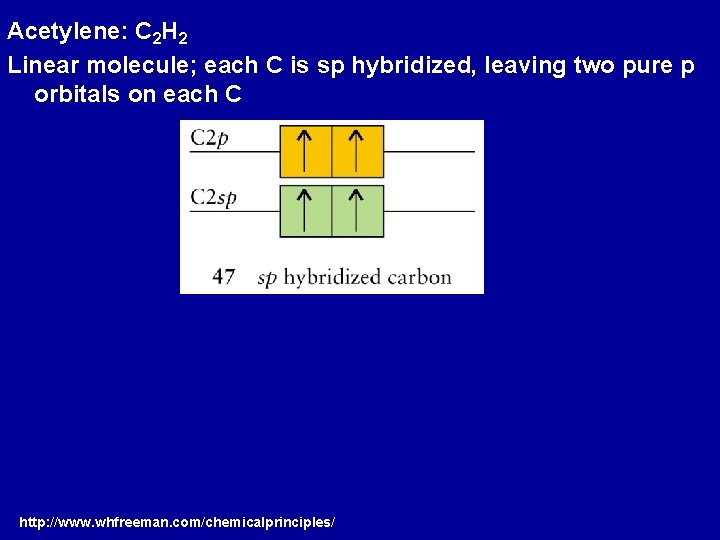
Acetylene: C 2 H 2 Linear molecule; each C is sp hybridized, leaving two pure p orbitals on each C http: //www. whfreeman. com/chemicalprinciples/

Multiple bonds are formed when an atom forms a s-bond by using an sp or sp 2 hybrid orbital and one or more p-bonds by using un-hybridized p orbitals

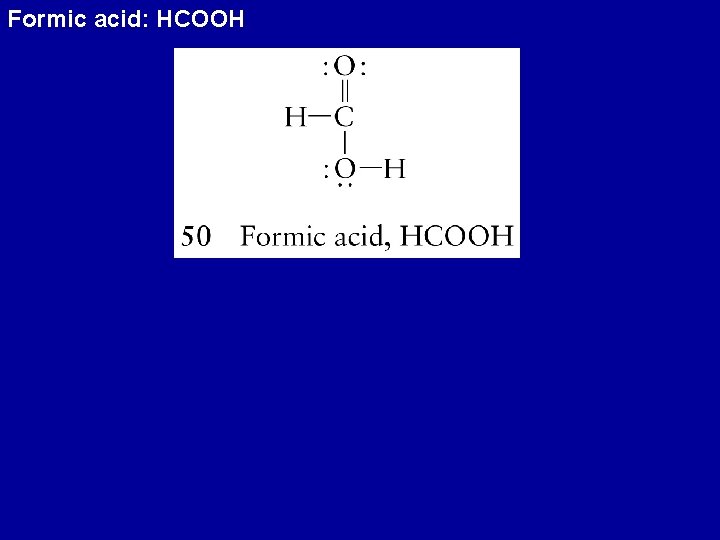
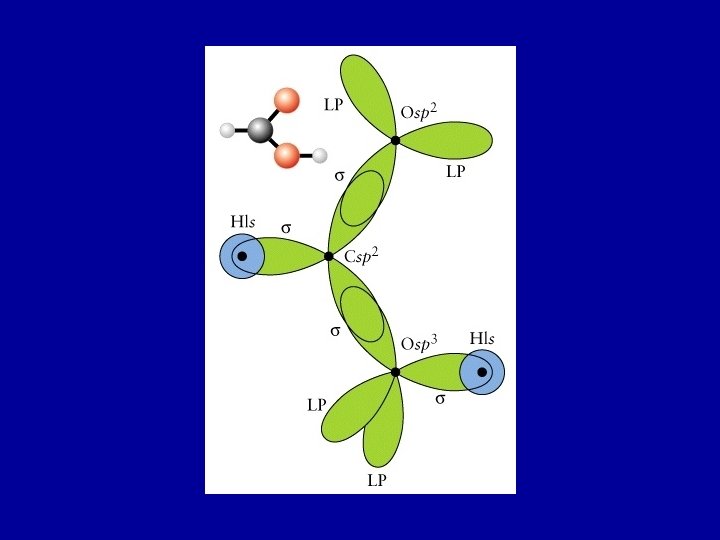
Formic acid: HCOOH
