HewittLyonsSuchockiYeh Conceptual Integrated Science Chapter 6 HEAT Copyright


























- Slides: 80

Hewitt/Lyons/Suchocki/Yeh Conceptual Integrated Science Chapter 6 HEAT Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

This lecture will help you understand: • • • • The Kinetic Theory of Matter Temperature Absolute Zero What Is Heat? Quantity of Heat The Laws of Thermodynamics Entropy Specific Heat Capacity Thermal Expansion of Water Heat Transfer: Conduction Heat Transfer: Convection Heat Transfer: Radiation Emission of Radiant Energy Absorption of Radiant Energy Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

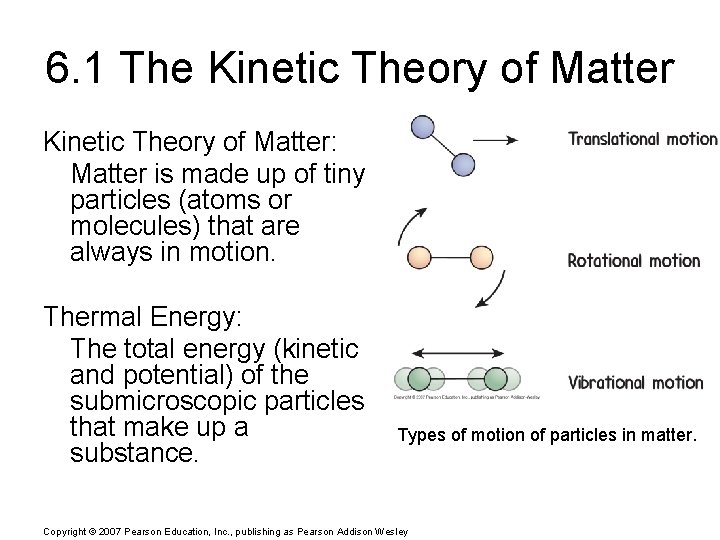
6. 1 The Kinetic Theory of Matter: Matter is made up of tiny particles (atoms or molecules) that are always in motion. Thermal Energy: The total energy (kinetic and potential) of the submicroscopic particles that make up a substance. Types of motion of particles in matter. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

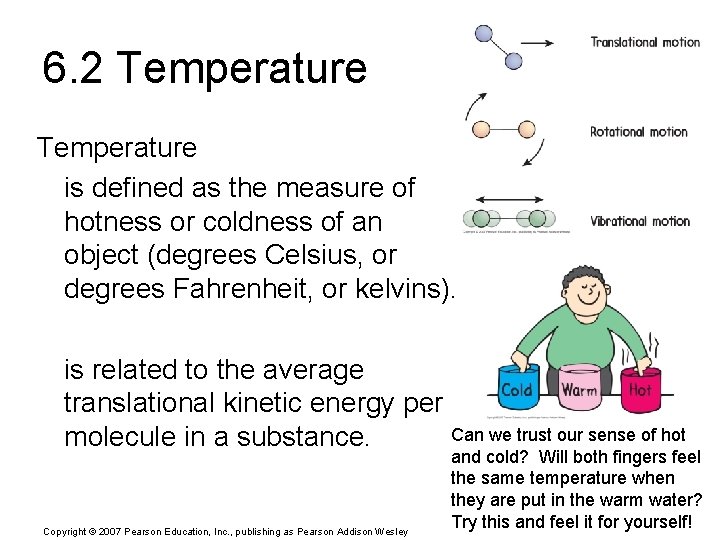
6. 2 Temperature is defined as the measure of hotness or coldness of an object (degrees Celsius, or degrees Fahrenheit, or kelvins). is related to the average translational kinetic energy per Can we trust our sense of hot molecule in a substance. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley and cold? Will both fingers feel the same temperature when they are put in the warm water? Try this and feel it for yourself!

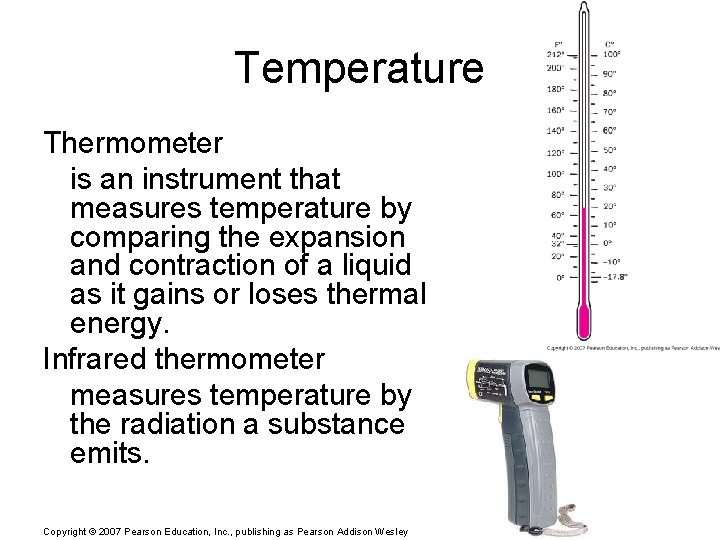
Temperature Thermometer is an instrument that measures temperature by comparing the expansion and contraction of a liquid as it gains or loses thermal energy. Infrared thermometer measures temperature by the radiation a substance emits. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

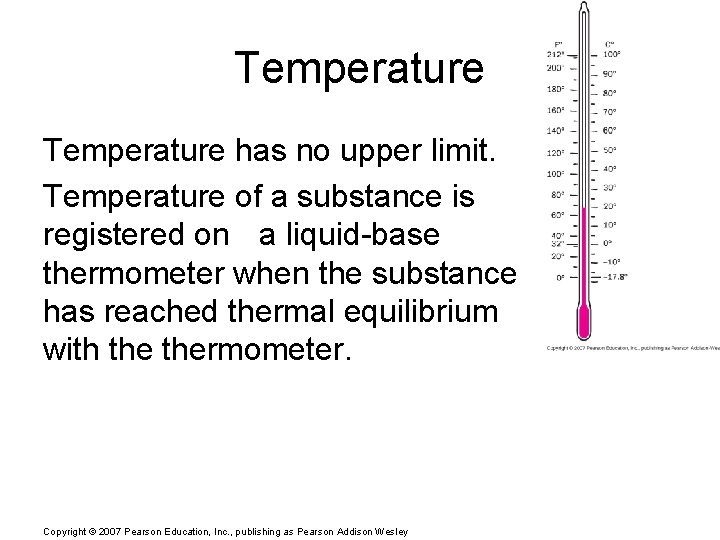
Temperature has no upper limit. Temperature of a substance is registered on a liquid-base thermometer when the substance has reached thermal equilibrium with thermometer. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

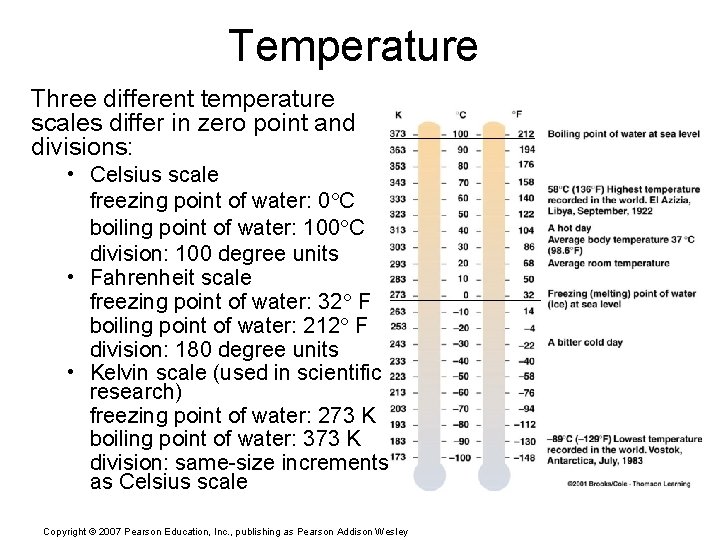
Temperature Three different temperature scales differ in zero point and divisions: • Celsius scale freezing point of water: 0 C boiling point of water: 100 C division: 100 degree units • Fahrenheit scale freezing point of water: 32 F boiling point of water: 212 F division: 180 degree units • Kelvin scale (used in scientific research) freezing point of water: 273 K boiling point of water: 373 K division: same-size increments as Celsius scale Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

Temperature CHECK YOUR NEIGHBOR There is twice as much molecular kinetic energy in 2 liters of boiling water as in 1 liter of boiling water. Which will be the same for both? A. B. C. D. Temperature. Thermal energy. Both of the above. None of the above. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

Temperature CHECK YOUR ANSWER There is twice as much molecular kinetic energy in 2 liters of boiling water as in 1 liter of boiling water. Which will be the same for both? A. B. C. D. Temperature. Thermal energy. Both of the above. None of the above. Explanation: Average kinetic energy of molecules is the same, which means temperature is the same for both. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

• One Kelvin is the same as one degree Celsius! Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

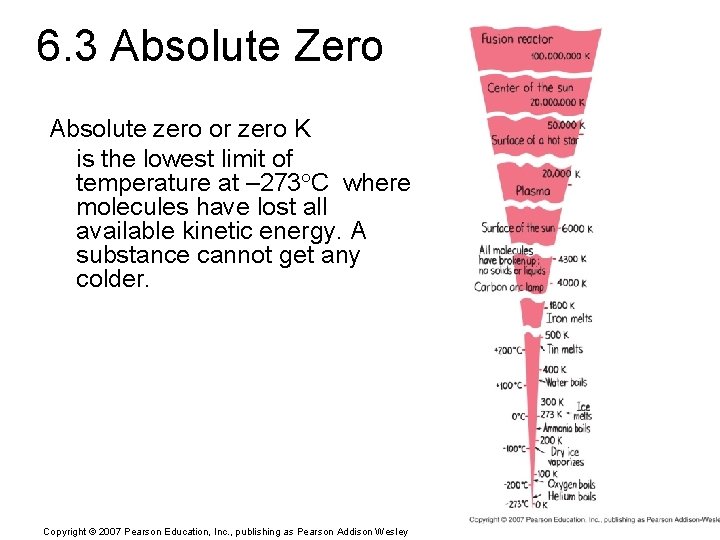
6. 3 Absolute Zero Absolute zero or zero K is the lowest limit of temperature at – 273 C where molecules have lost all available kinetic energy. A substance cannot get any colder. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

6. 4 What Is Heat? Heat is defined as a flow of thermal energy due to a temperature difference. The direction of heat flow is from a higher-temperature substance to a lower-temperature substance. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

• The temperature of the sparks is very high, about 2000°C. That’s a lot of thermal energy per molecule of spark. However, because there are few molecules per spark, internal energy is safely small. Temperature is one thing; transfer of energy is another. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

What Is Heat? CHECK YOUR NEIGHBOR If a red hot thumbtack is immersed in warm water, the direction of heat flow will be from the A. B. C. D. warm water to the red hot thumbtack to the warm water. no heat flow. not enough information. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

What Is Heat? CHECK YOUR ANSWER If a red hot thumbtack is immersed in warm water, the direction of heat flow will be from the A. B. C. D. warm water to the red hot thumbtack to the warm water. no heat flow. not enough information. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

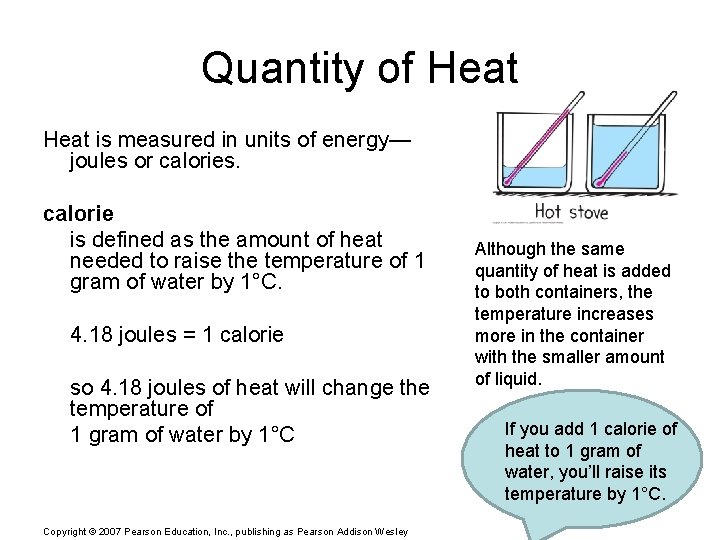
Quantity of Heat is measured in units of energy— joules or calories. calorie is defined as the amount of heat needed to raise the temperature of 1 gram of water by 1°C. 4. 18 joules = 1 calorie so 4. 18 joules of heat will change the temperature of 1 gram of water by 1°C Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley Although the same quantity of heat is added to both containers, the temperature increases more in the container with the smaller amount of liquid. If you add 1 calorie of heat to 1 gram of water, you’ll raise its temperature by 1°C.

Quantity of Heat Energy rating of food and fuel is measured by energy released when they are metabolized. Kilocalorie: Heat unit for labeling food One kilocalorie or Calorie (with a capital C) is the heat needed to change the temperature of 1 kilogram of water by 1°C. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley To the weight watcher, the peanut contains 10 Calories, to the physicist, it releases 10, 000 calories (or 41, 840 joules) of energy when burned or digested.

Quantity of Heat CHECK YOUR NEIGHBOR The same quantity of heat is added to different amounts of water in two equal-size containers. The temperature of the smaller amount of water A. B. C. D. decreases more. increases more. does not change. not enough information. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

Quantity of Heat CHECK YOUR ANSWER The same quantity of heat is added to different amounts of water in two equal-size containers. The temperature of the smaller amount of water A. B. C. D. decreases more. increases more. does not change. not enough information. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

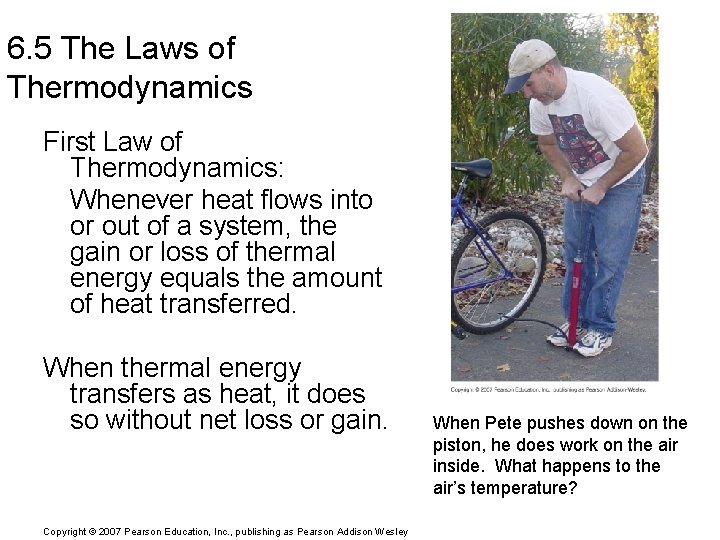
6. 5 The Laws of Thermodynamics First Law of Thermodynamics: Whenever heat flows into or out of a system, the gain or loss of thermal energy equals the amount of heat transferred. When thermal energy transfers as heat, it does so without net loss or gain. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley When Pete pushes down on the piston, he does work on the air inside. What happens to the air’s temperature?

The Laws of Thermodynamics CHECK YOUR NEIGHBOR When work is done on a system, compressing air in a tire pump, for example, the temperature of the system A. B. C. D. increases. decreases. remains unchanged. is no longer evident. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

The Laws of Thermodynamics CHECK YOUR ANSWER When work is done on a system, compressing air in a tire pump, for example, the temperature of the system A. B. C. D. increases. decreases. remains unchanged. is no longer evident. Explanation: In accord with the first law of thermodynamics, work input increases the energy of the system. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

The Laws of Thermodynamics Second Law of Thermodynamics: Heat never spontaneously flows from a lower-temperature substance to a highertemperature substance. Heat can be made to flow the opposite way only when work is done on the system or by adding energy from another source. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

The Laws of Thermodynamics Third Law of Thermodynamics: No system can reach absolute zero. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

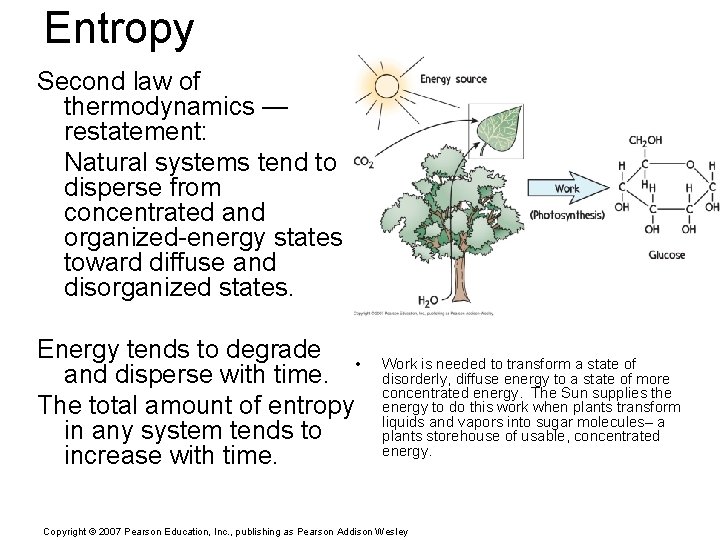
Entropy is a measure of the disorder of a system. Whenever energy freely transforms from one form to another, the direction of transformation is toward a state of greater disorder and, therefore, toward one of greater entropy. The greater the disorder the higher the entropy. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

Entropy Second law of thermodynamics — restatement: Natural systems tend to disperse from concentrated and organized-energy states toward diffuse and disorganized states. Energy tends to degrade • and disperse with time. The total amount of entropy in any system tends to increase with time. Work is needed to transform a state of disorderly, diffuse energy to a state of more concentrated energy. The Sun supplies the energy to do this work when plants transform liquids and vapors into sugar molecules– a plants storehouse of usable, concentrated energy. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

Entropy CHECK YOUR NEIGHBOR Your room gets messier each week. In this case, the entropy of your room is A. B. C. D. increasing. decreasing. hanging steady. nonexistent. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

Entropy CHECK YOUR ANSWER Your room gets messier each week. In this case, the entropy of your room is A. B. C. D. increasing. decreasing. hanging steady. nonexistent. Comment: If your room became more organized each week, then entropy would decrease in proportion to the effort expended. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

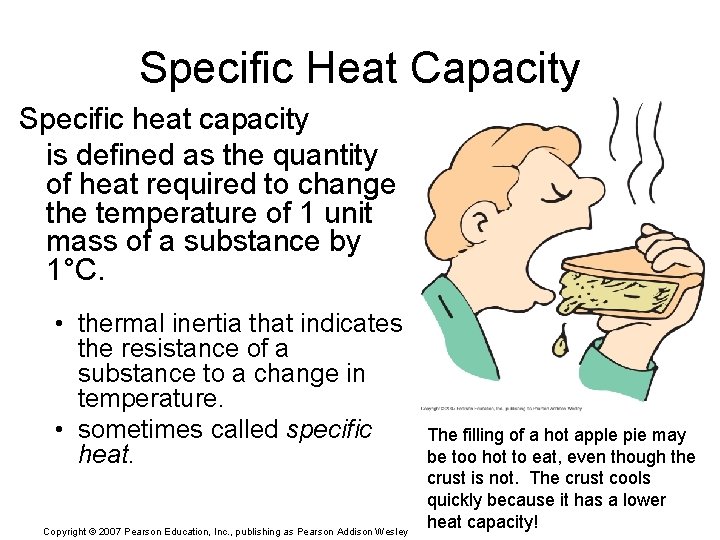
Specific Heat Capacity Specific heat capacity is defined as the quantity of heat required to change the temperature of 1 unit mass of a substance by 1°C. • thermal inertia that indicates the resistance of a substance to a change in temperature. • sometimes called specific heat. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley The filling of a hot apple pie may be too hot to eat, even though the crust is not. The crust cools quickly because it has a lower heat capacity!

Specific Heat CHECK YOUR NEIGHBOR Which has the higher specific heat, water or land? A. B. C. D. Water. Land. Both of the above are the same. None of the above. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

Specific Heat CHECK YOUR ANSWER Which has the higher specific heat, water or land? A. B. C. D. Water. Land. Both of the above are the same. None of the above. Explanation: A substance with small temperature changes for large heat changes has a high specific heat capacity. Water takes much longer to heat up in the sunshine than does land. This difference is a major influence on climate. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley



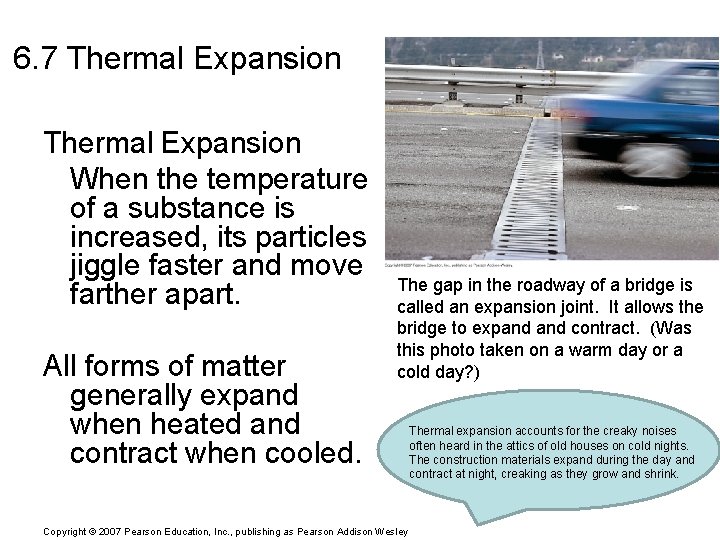
6. 7 Thermal Expansion When the temperature of a substance is increased, its particles jiggle faster and move farther apart. All forms of matter generally expand when heated and contract when cooled. The gap in the roadway of a bridge is called an expansion joint. It allows the bridge to expand contract. (Was this photo taken on a warm day or a cold day? ) Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley Thermal expansion accounts for the creaky noises often heard in the attics of old houses on cold nights. The construction materials expand during the day and contract at night, creaking as they grow and shrink.

• . . Videos for class�6_How. AThermostat. Wo_VID. mov Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

Thermal Expansion CHECK YOUR NEIGHBOR When stringing telephone lines between poles in the summer, it is advisable to allow the lines to A. B. C. D. sag. be taut. be close to the ground. allow ample space for birds. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

Thermal Expansion CHECK YOUR ANSWER When stringing telephone lines between poles in the summer, it is advisable to allow the lines to A. B. C. D. sag. be taut. be close to the ground. allow ample space for birds. Explanation: Telephone lines are longer in a warmer summer and shorter in a cold winter. Hence, they sag more on hot summer days than in winter. If the lines are not strung with enough sag in summer, they might contract too much and snap during the winter—especially when carrying ice. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

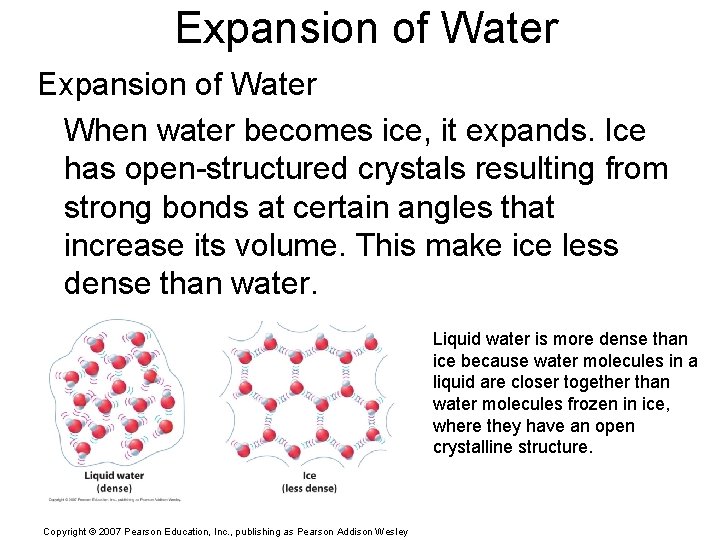
Expansion of Water When water becomes ice, it expands. Ice has open-structured crystals resulting from strong bonds at certain angles that increase its volume. This make ice less dense than water. Liquid water is more dense than ice because water molecules in a liquid are closer together than water molecules frozen in ice, where they have an open crystalline structure. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

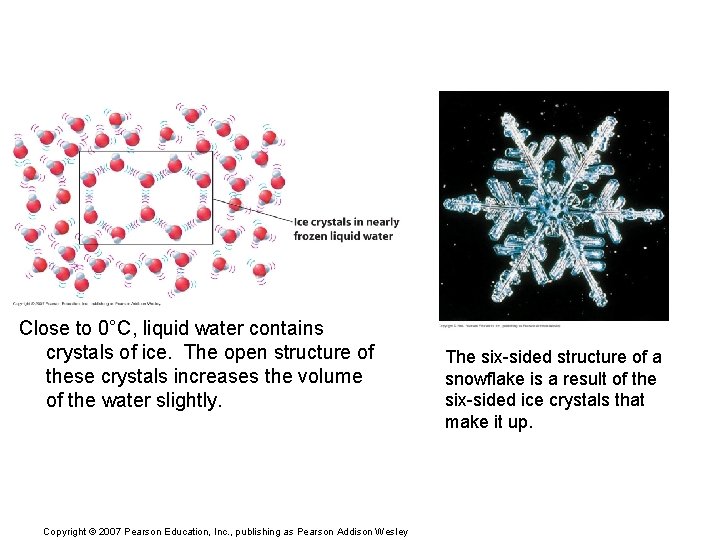
Close to 0°C, liquid water contains crystals of ice. The open structure of these crystals increases the volume of the water slightly. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley The six-sided structure of a snowflake is a result of the six-sided ice crystals that make it up.

Expansion of Water between 0 C and 4 C does not expand with temperature. As the temperature of 0 water rises, it contracts until it reaches 4 C. Thereafter, it expands. Water is at its smallest volume and greatest density at 4 C. When 0 C water freezes to become ice, however, it has its largest volume and lowest density. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

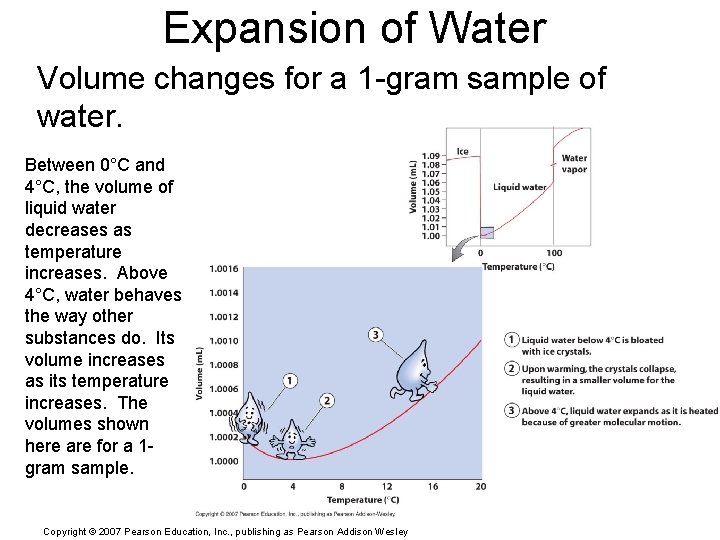
Expansion of Water Volume changes for a 1 -gram sample of water. Between 0°C and 4°C, the volume of liquid water decreases as temperature increases. Above 4°C, water behaves the way other substances do. Its volume increases as its temperature increases. The volumes shown here are for a 1 gram sample. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

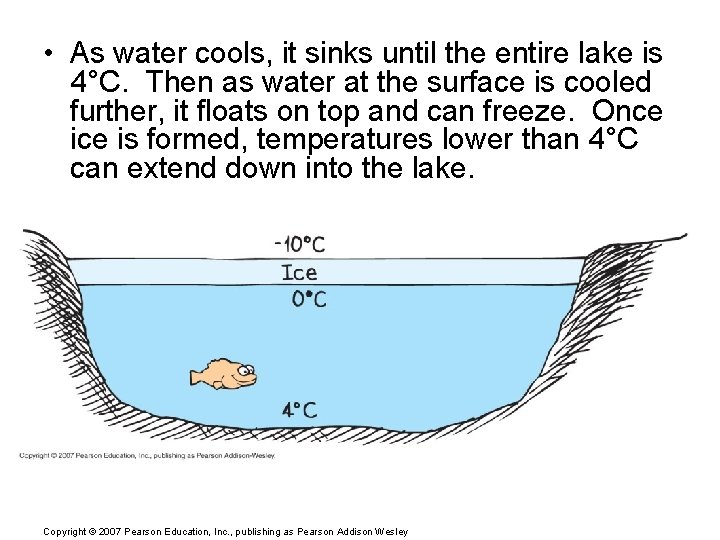
• As water cools, it sinks until the entire lake is 4°C. Then as water at the surface is cooled further, it floats on top and can freeze. Once is formed, temperatures lower than 4°C can extend down into the lake. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

• . . Videos for class�6_Low. Temperatures. Ni_VID. mov Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

Expansion of Water CHECK YOUR NEIGHBOR When a sample of 0 C water is heated, it first A. B. C. D. expands. contracts. remains unchanged. not enough information. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

Expansion of Water CHECK YOUR ANSWER When a sample of 0 C water is heated, it first A. B. C. D. expands. contracts. remains unchanged. not enough information. Explanation: Water continues to contract until it reaches a temperature of 4 C. With further increase in temperature beyond 4 C, water then expands. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

Expansion of Water CHECK YOUR NEIGHBOR When a sample of 4 C water is cooled, it A. B. C. D. expands. contracts. remains unchanged. not enough information. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

Expansion of Water CHECK YOUR ANSWER When a sample of 4 C water is cooled, it A. B. C. D. expands. contracts. remains unchanged. not enough information. Explanation: Parts of the water will crystallize and occupy more space. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

6. 8 Heat Transfer Processes of thermal energy transfer: • conduction • convection • radiation Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

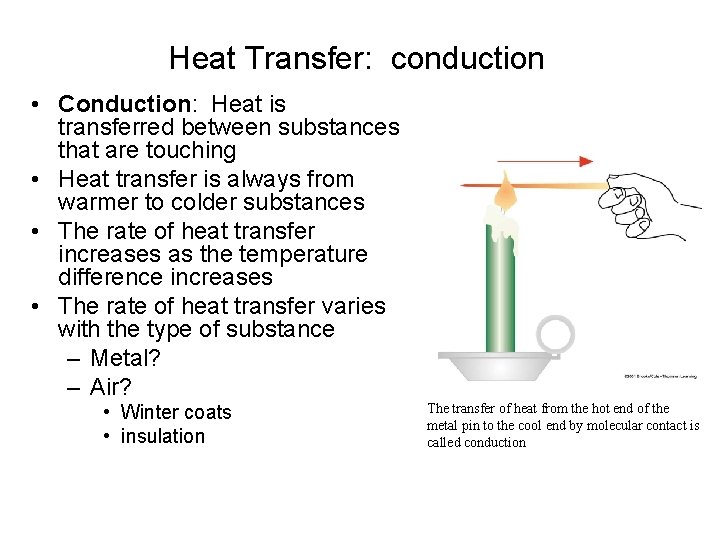
Heat Transfer: conduction • Conduction: Heat is transferred between substances that are touching • Heat transfer is always from warmer to colder substances • The rate of heat transfer increases as the temperature difference increases • The rate of heat transfer varies with the type of substance – Metal? – Air? • Winter coats • insulation The transfer of heat from the hot end of the metal pin to the cool end by molecular contact is called conduction

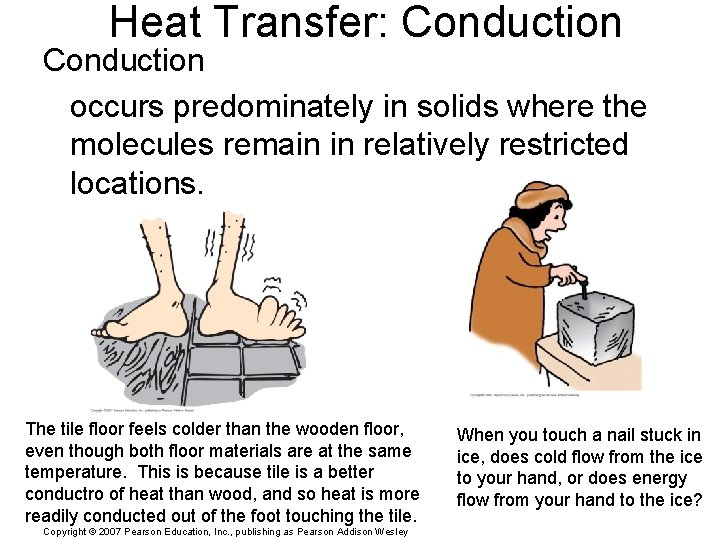
Heat Transfer: Conduction occurs predominately in solids where the molecules remain in relatively restricted locations. The tile floor feels colder than the wooden floor, even though both floor materials are at the same temperature. This is because tile is a better conductro of heat than wood, and so heat is more readily conducted out of the foot touching the tile. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley When you touch a nail stuck in ice, does cold flow from the ice to your hand, or does energy flow from your hand to the ice?

Heat Transfer: Conduction Example: When one end of a solid is placed near a heat source, electrons and adjacent molecules gain kinetic energy and start to move faster and farther. They collide with neighboring molecules and transfer some of their kinetic energy to them. These molecules then interact with other neighboring molecules, and thermal energy is gradually transferred along the solid. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

Heat Transfer: Conduction Good conductors: • composed of atoms with “loose” outer electrons • known as poor insulators • examples—all metals to varying degrees Poor conductors: • delay the transfer of heat • known as good insulators • examples—wood, wool, straw, paper, cork, Styrofoam, liquid, gases, air, or materials with trapped air Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

Heat Transfer: Conduction No insulator can totally prevent heat from getting through it. An insulator reduces the rate at which heat penetrates. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

• . . Videos for class�6_The. Secret. To. Walki_VID. mov Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

• Firewalking author John Suchocki isn’t burned by the red-hot wooden coals due to the low heat conductivity of wood Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

Heat Transfer: Conduction CHECK YOUR NEIGHBOR If you hold one end of a metal bar against a piece of ice, the end in your hand will soon become cold. Does cold flow from the ice to your hand? A. B. C. D. Yes. In some cases, yes. No. In some cases, no. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

Heat Transfer: Conduction CHECK YOUR ANSWER If you hold one end of a metal bar against a piece of ice, the end in your hand will soon become cold. Does cold flow from the ice to your hand? A. B. C. D. Yes. In some cases, yes. No. In some cases, no. Explanation: Cold does not flow from the ice to your hand. Heat flows from your hand to the ice. The metal is cold to your touch, because you are transferring heat to the metal. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

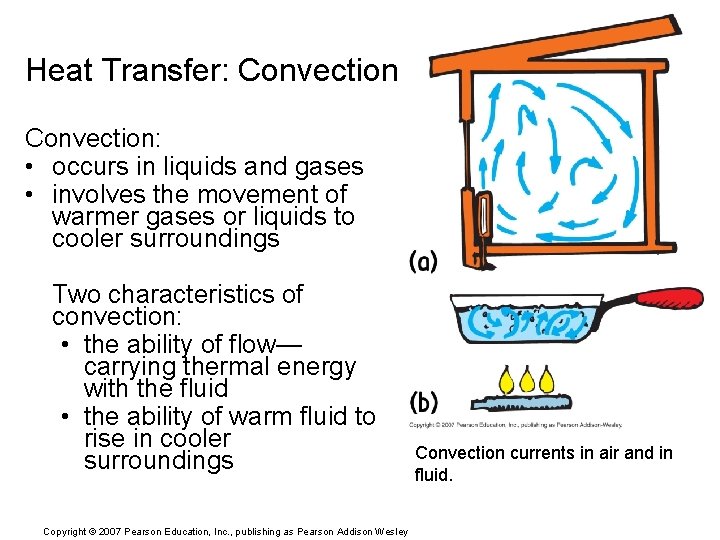
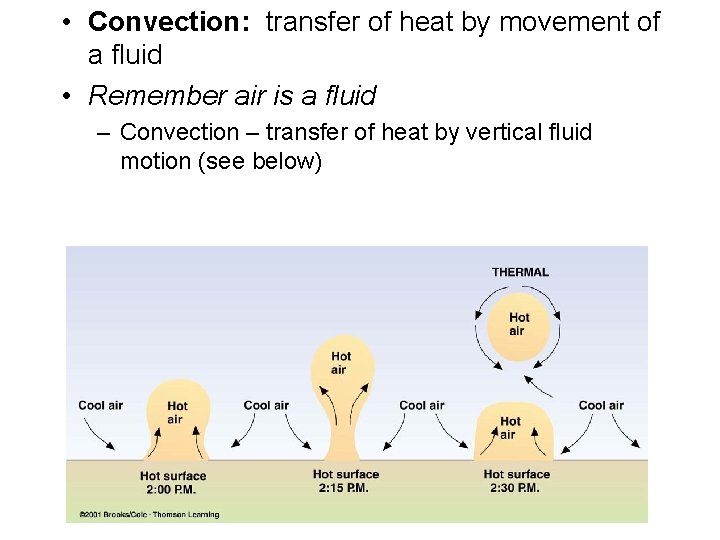
Heat Transfer: Convection: • occurs in liquids and gases • involves the movement of warmer gases or liquids to cooler surroundings Two characteristics of convection: • the ability of flow— carrying thermal energy with the fluid • the ability of warm fluid to rise in cooler surroundings Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley Convection currents in air and in fluid.

• Convection: transfer of heat by movement of a fluid • Remember air is a fluid – Convection – transfer of heat by vertical fluid motion (see below)

• Although we can’t see air, there are signs that tell us where the air is rising. On a calm day, you can watch a hawk circle and climb high above level ground while its wings remain motionless. A rising thermal carries the hawk upward as it scans the terrain for prey. If the water vapor inside the rising thermal condenses into liquid cloud droplets, thermal becomes visible to us as a puffy cumulus cloud. Flying in a light aircraft beneath these clouds usually produces a bumpy ride, as passengers are jostled around by the rising and sinking air associated with convection.

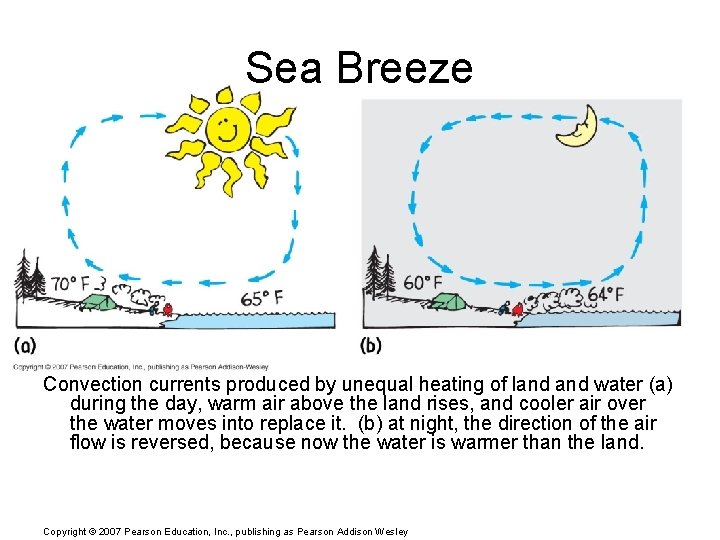
Sea Breeze Convection currents produced by unequal heating of land water (a) during the day, warm air above the land rises, and cooler air over the water moves into replace it. (b) at night, the direction of the air flow is reversed, because now the water is warmer than the land. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

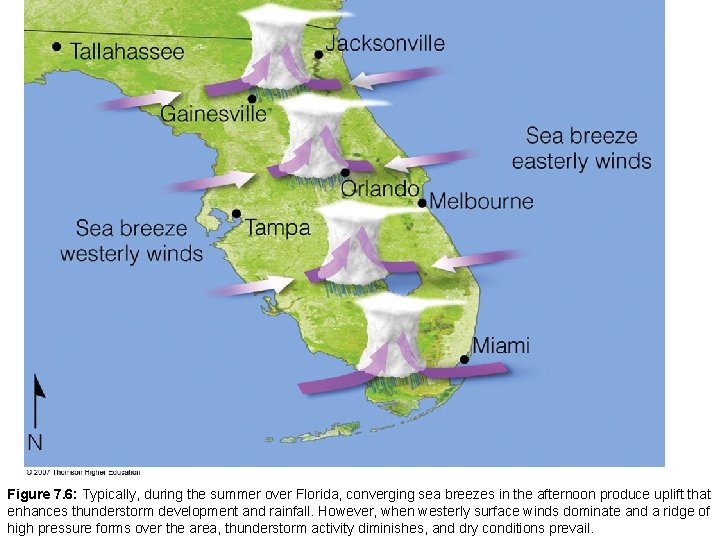
Surface heating and lifting of air along a sea breeze combine to form thunderstorms almost daily during the summer in southern Florida.

Figure 7. 6: Typically, during the summer over Florida, converging sea breezes in the afternoon produce uplift that enhances thunderstorm development and rainfall. However, when westerly surface winds dominate and a ridge of high pressure forms over the area, thunderstorm activity diminishes, and dry conditions prevail.

Land Sea Breeze animations • http: //www. classzone. com/books/earth_science/t erc/content/visualizations/es 1903 page 01. cfm? chapter_no=visualization • Satellite of sea breeze: http: //www. met. tamu. edu/class/atmo 151/tut/seab r/sea 18. html • Lake breeze http: //itg 1. meteor. wisc. edu/wxwise/Ackerman. Kn ox/chap 12/great_lake_breeze. html

Heat Transfer: Convection CHECK YOUR NEIGHBOR Although warm air rises, why are mountaintops cold and snow covered, while the valleys below are relatively warm and green? A. B. C. D. Warm air cools when rising. There is a thick insulating blanket of air above valleys. Both of the above. None of the above. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

Heat Transfer: Convection CHECK YOUR ANSWER Although warm air rises, why are mountaintops cold and snow covered, while the valleys below are relatively warm and green? A. B. C. D. Warm air cools when rising. There is a thick insulating blanket of air above valleys. Both of the above. None of the above. Explanation: Earth’s atmosphere acts as a blanket, which for one important thing, keeps Earth from freezing at nighttime. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

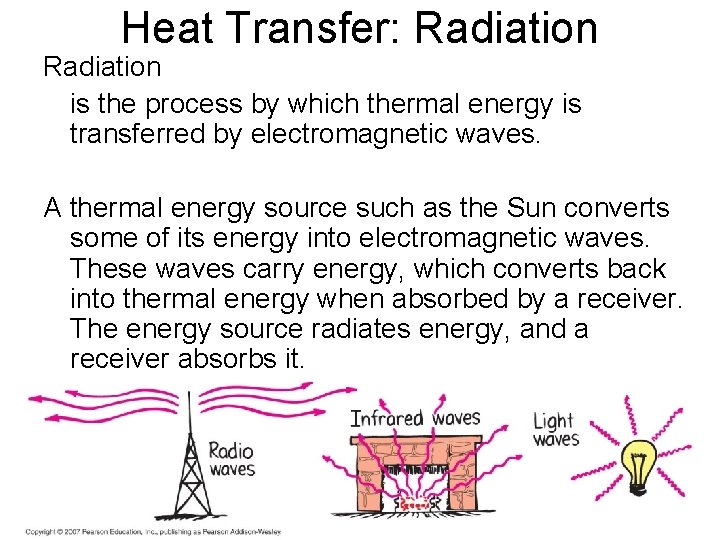
Heat Transfer: Radiation is the process by which thermal energy is transferred by electromagnetic waves. A thermal energy source such as the Sun converts some of its energy into electromagnetic waves. These waves carry energy, which converts back into thermal energy when absorbed by a receiver. The energy source radiates energy, and a receiver absorbs it. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

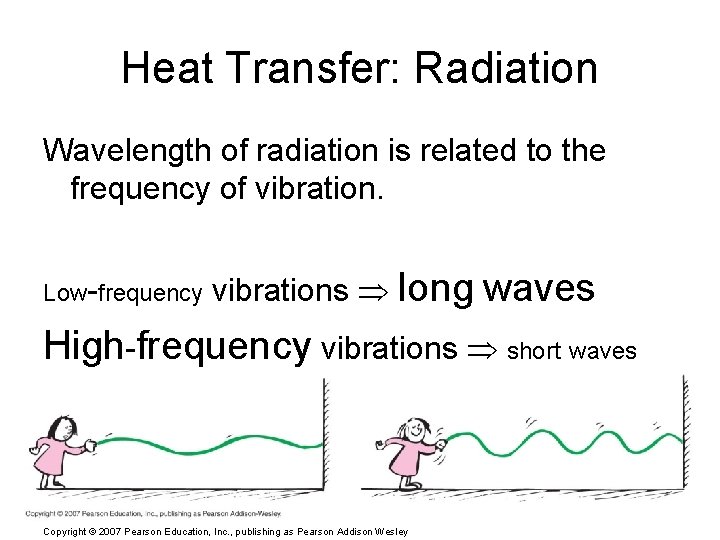
Heat Transfer: Radiation Wavelength of radiation is related to the frequency of vibration. Low-frequency vibrations long waves High-frequency vibrations short waves Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

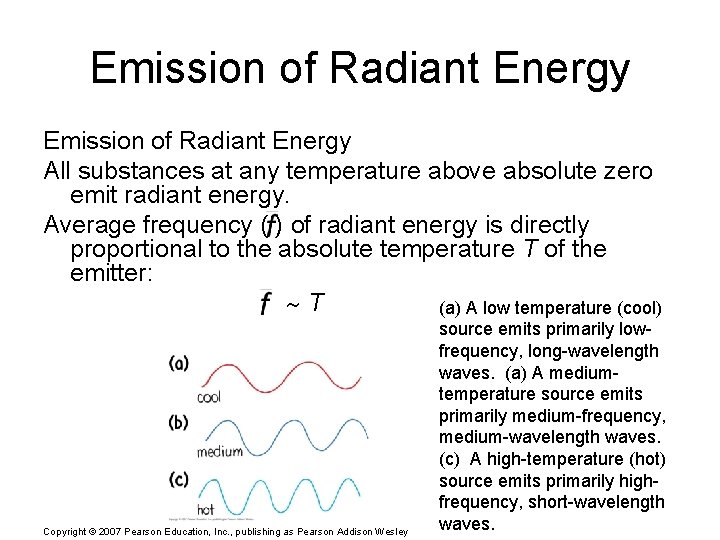
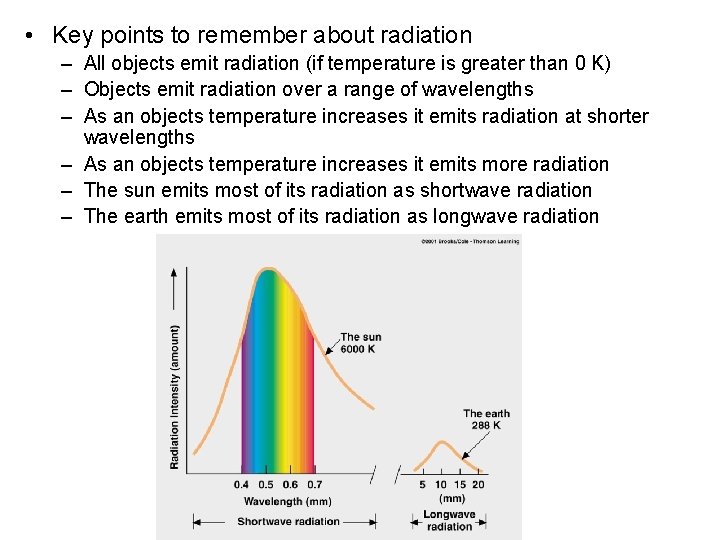
Emission of Radiant Energy All substances at any temperature above absolute zero emit radiant energy. Average frequency ( ) of radiant energy is directly proportional to the absolute temperature T of the emitter: T (a) A low temperature (cool) Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley source emits primarily lowfrequency, long-wavelength waves. (a) A mediumtemperature source emits primarily medium-frequency, medium-wavelength waves. (c) A high-temperature (hot) source emits primarily highfrequency, short-wavelength waves.

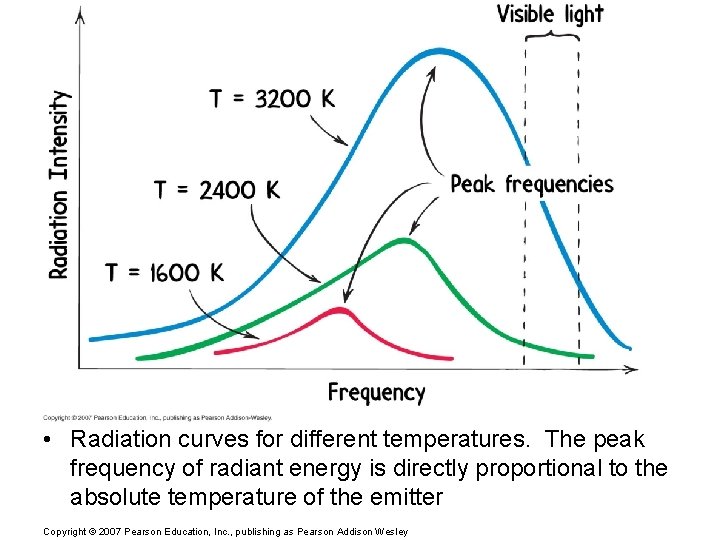
• Radiation curves for different temperatures. The peak frequency of radiant energy is directly proportional to the absolute temperature of the emitter Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

• Both the Sun and the Earth emit radiant energy. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

• Key points to remember about radiation – All objects emit radiation (if temperature is greater than 0 K) – Objects emit radiation over a range of wavelengths – As an objects temperature increases it emits radiation at shorter wavelengths – As an objects temperature increases it emits more radiation – The sun emits most of its radiation as shortwave radiation – The earth emits most of its radiation as longwave radiation

• A closer look at the radiation emitted by the sun:

Emission of Radiant Energy CHECK YOUR NEIGHBOR If a good absorber of radiant energy were a poor emitter, its temperature compared with its surroundings would be A. B. C. D. lower. higher. unaffected. none of the above. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

Emission of Radiant Energy CHECK YOUR ANSWER If a good absorber of radiant energy were a poor emitter, its temperature compared with its surroundings would be A. B. C. D. lower. higher. unaffected. none of the above. Explanation: If a good absorber were not also a good emitter, there would be a net absorption of radiant energy, and the temperature of a good absorber would remain higher than the temperature of the surroundings. Nature is not so! Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

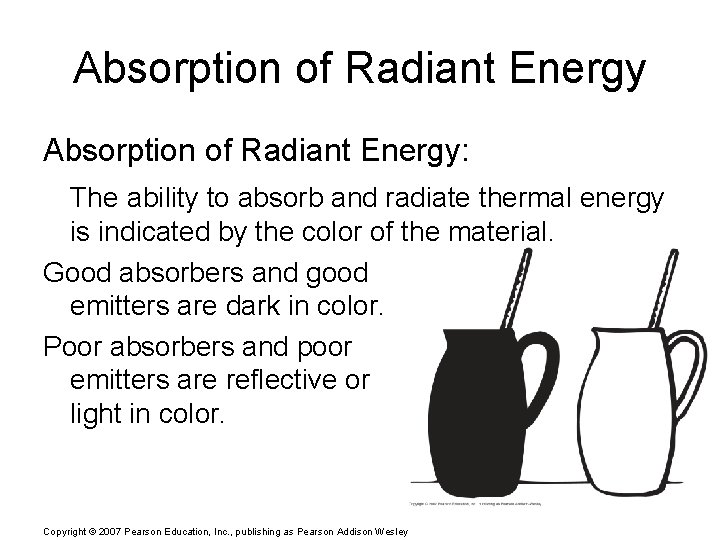
Absorption of Radiant Energy: The ability to absorb and radiate thermal energy is indicated by the color of the material. Good absorbers and good emitters are dark in color. Poor absorbers and poor emitters are reflective or light in color. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

Absorption of Radiant Energy The surface of any material both absorbs and emits radiant energy. When a surface absorbs more energy than it emits, it is a net absorber, and temperature rises. When a surface emits more energy than it absorbs, it is a net emitter, and temperature falls. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

Absorption of Radiant Energy Whether a surface is a net absorber or net emitter depends on whether its temperature is above or below that of its surroundings. A surface hotter than its surroundings will be a net emitter and will cool. A surface colder than its surroundings will be a net absorber and will warm. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

Absorption of Radiant Energy CHECK YOUR NEIGHBOR Which melts faster in sunshine—dirty snow or clean snow? A. B. C. D. Dirty snow. Clean snow. Both of the above. None of the above. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley

Absorption of Radiant Energy CHECK YOUR ANSWER Which melts faster in sunshine—dirty snow or clean snow? A. B. C. D. Dirty snow. Clean snow. Both of the above. None of the above. Explanation: Dirty snow absorbs more sunlight, whereas clean snow reflects more. Copyright © 2007 Pearson Education, Inc. , publishing as Pearson Addison Wesley