Heterogeneous Mixtures 1 Suspensions n n n Suspension







- Slides: 12

Heterogeneous Mixtures

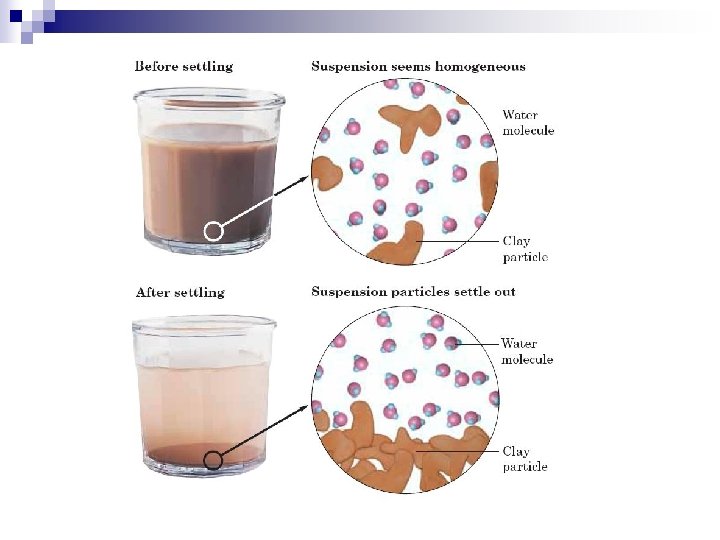
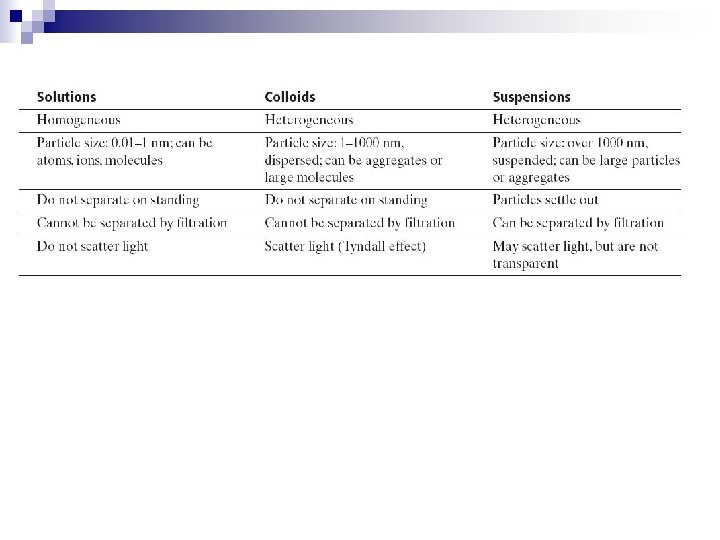
1. Suspensions n n n Suspension – a mixture containing particles that settle out if left undisturbed. Particles are too large to be dissolved. Particles are greater than 1000 nm in diameter. They can be filtered. Examples : muddy water, many “liquid” medicines that require “shaking, ”


2. Colloids n n n Colloid – Heterogeneous mixture of intermediate size particles. They do not dissolve or settle. These particles remain dispersed in the solution, they “float” and are suspended. Particles cannot be removed by filtering. They are between 1 nm and 1000 nm in diameter. The most abundant substance in a colloid is the dispersion medium.

n n n If you stir an electrolyte into the colloid, it will cause the particles to settle out. “Flocculents” bring the colloid particles together and the mass becomes so great that it forms a suspension. Examples of colloids include smog, fog, milk, mayonnaise, marshmallow, smoke, clouds.

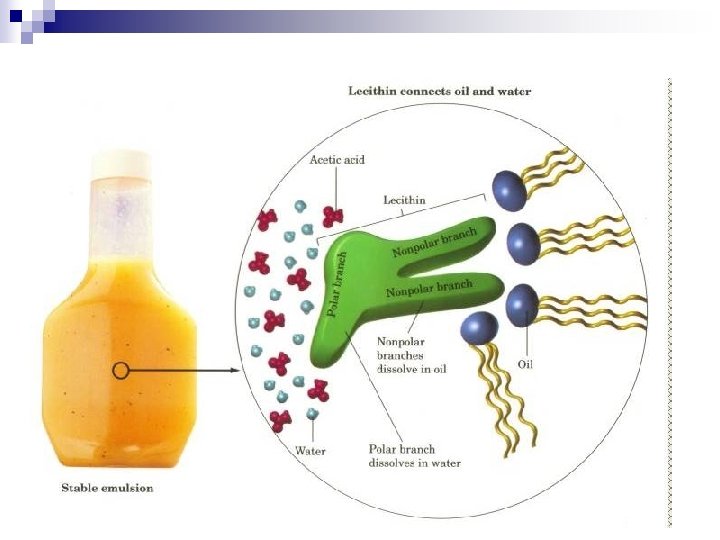
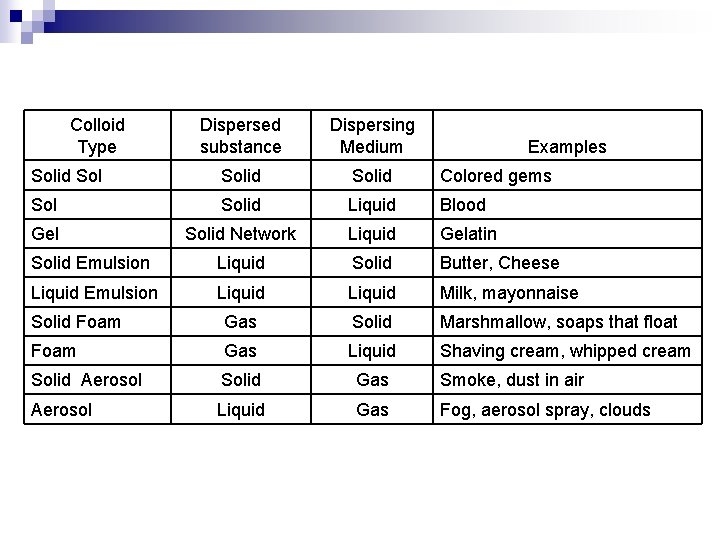
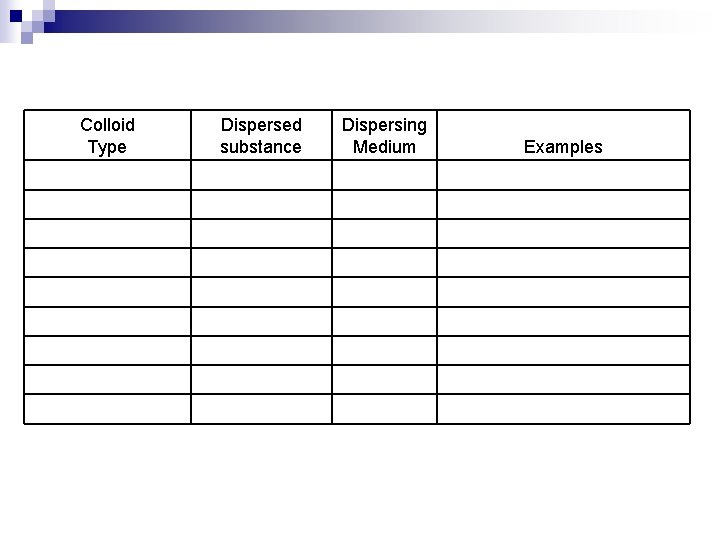
n n n Colloids are classified as : sol, gels, emulsions, foam, and aerosols. Sol – a solid dispersed in a liquid ¨ Examples : paints, blood Gel – a solid network extending throughout a liquid. ¨ Example : gelatin (jello) Liquid emulsion – liquid dispersed in a liquid ¨ Examples : milk, mayonnaise Solid emulsion – liquid dispersed in a solid ¨ Examples : cheese, butter


n n Foam – gas dispersed in liquid ¨ Examples : shaving cream, whipped cream, beaten egg white Solid Foam – Gas dispersed in a solid ¨ Examples : marshmallow, soaps that float Solid aerosol – solid dispersed in gas (air) ¨ Examples : smoke, air borne particulate matter, auto exhaust Liquid aerosol – liquid dispersed in gas (air) ¨ Examples : fog, mist, clouds, aerosol spray

n n n Brownian motion – the random, erratic motion of colloid particles. ¨ Caused by the polar areas of the molecules. They attract and repel each other, keeping them in constant motion. Tyndall Effect – particles in a colloid are large enough to scatter light. Turbidity – how cloudy the water is.


Colloid Type Dispersed substance Dispersing Medium Solid Colored gems Solid Liquid Blood Gel Solid Network Liquid Gelatin Solid Emulsion Liquid Solid Butter, Cheese Liquid Emulsion Liquid Milk, mayonnaise Solid Foam Gas Solid Marshmallow, soaps that float Foam Gas Liquid Shaving cream, whipped cream Solid Aerosol Solid Gas Smoke, dust in air Aerosol Liquid Gas Fog, aerosol spray, clouds Examples

Colloid Type Dispersed substance Dispersing Medium Examples