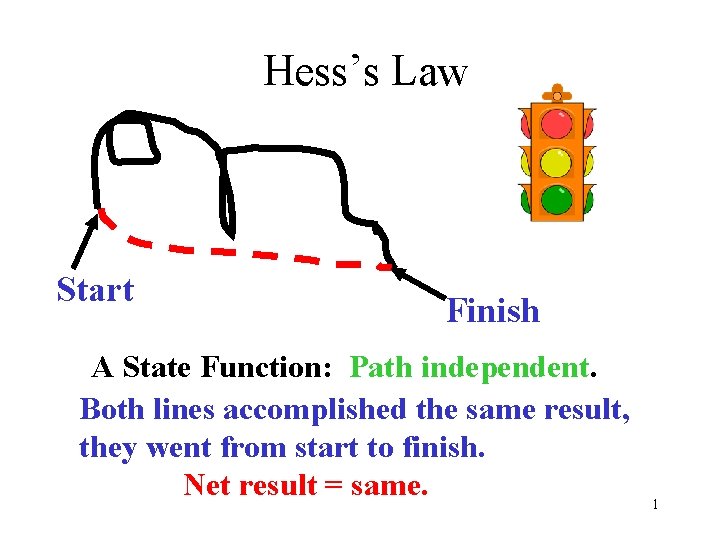
Hesss Law Start Finish A State Function Path
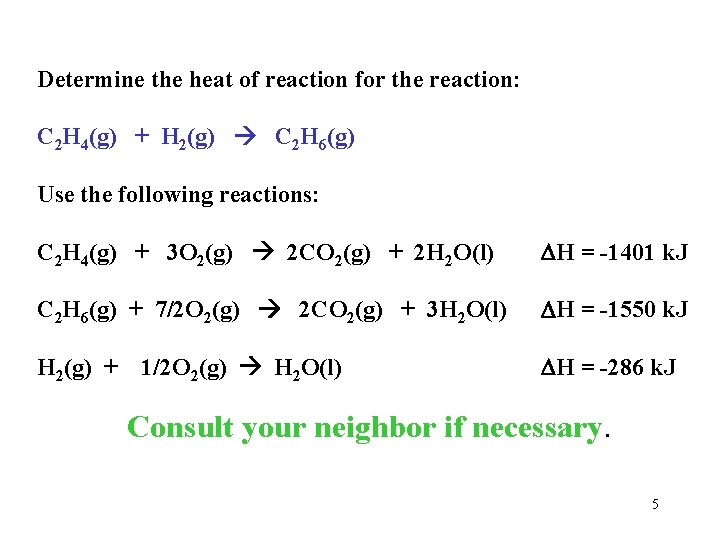

- Slides: 10

Hess’s Law Start Finish A State Function: Path independent. Both lines accomplished the same result, they went from start to finish. Net result = same. 1

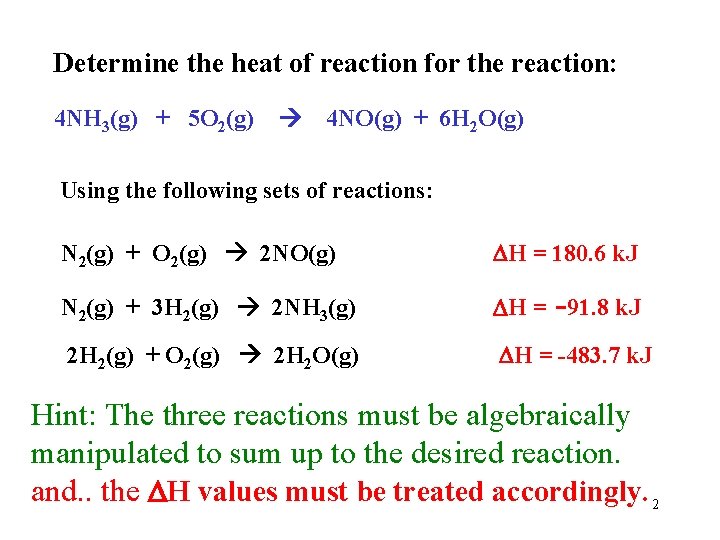
Determine the heat of reaction for the reaction: 4 NH 3(g) + 5 O 2(g) 4 NO(g) + 6 H 2 O(g) Using the following sets of reactions: N 2(g) + O 2(g) 2 NO(g) H = 180. 6 k. J N 2(g) + 3 H 2(g) 2 NH 3(g) H = -91. 8 k. J 2 H 2(g) + O 2(g) 2 H 2 O(g) H = -483. 7 k. J Hint: The three reactions must be algebraically manipulated to sum up to the desired reaction. and. . the H values must be treated accordingly. 2

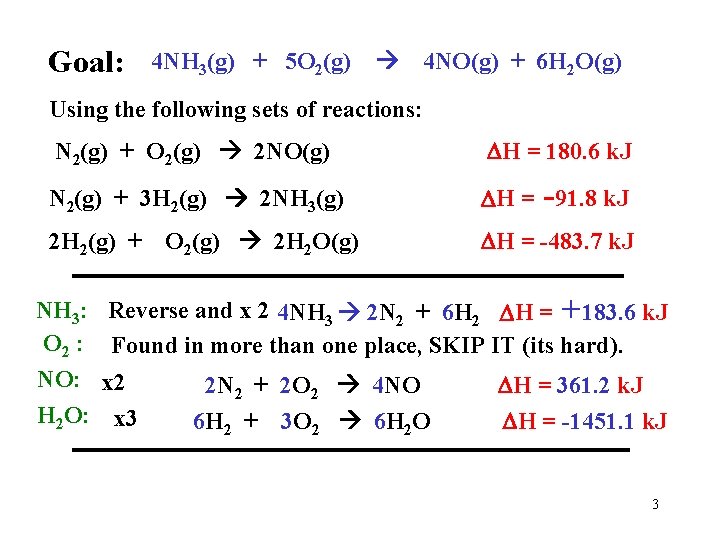
Goal: 4 NH 3(g) + 5 O 2(g) 4 NO(g) + 6 H 2 O(g) Using the following sets of reactions: N 2(g) + O 2(g) 2 NO(g) H = 180. 6 k. J N 2(g) + 3 H 2(g) 2 NH 3(g) H = -91. 8 k. J 2 H 2(g) + NH 3: O 2 : NO: H 2 O: O 2(g) 2 H 2 O(g) H = -483. 7 k. J Reverse and x 2 4 NH 3 2 N 2 + 6 H 2 H = +183. 6 k. J Found in more than one place, SKIP IT (its hard). x 2 x 3 2 N 2 + 2 O 2 4 NO 6 H 2 + 3 O 2 6 H 2 O H = 361. 2 k. J H = -1451. 1 k. J 3

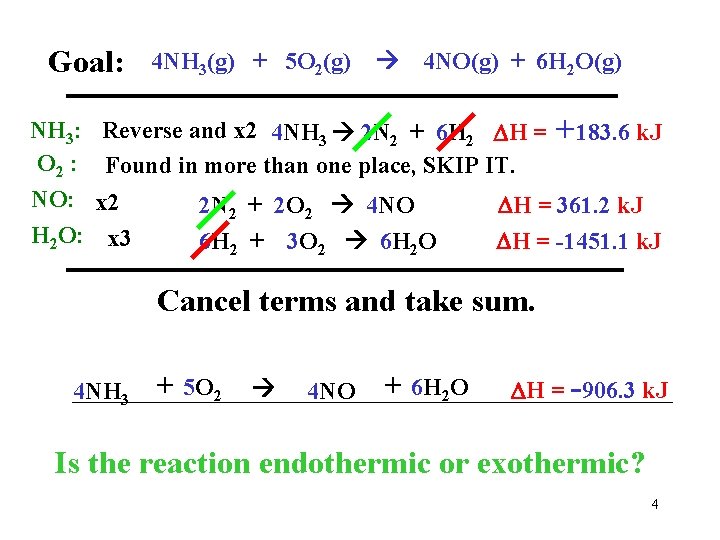
Goal: NH 3: O 2 : NO: H 2 O: 4 NH 3(g) + 5 O 2(g) 4 NO(g) + 6 H 2 O(g) Reverse and x 2 4 NH 3 2 N 2 + 6 H 2 H = Found in more than one place, SKIP IT. x 2 x 3 2 N 2 + 2 O 2 4 NO 6 H 2 + 3 O 2 6 H 2 O +183. 6 k. J H = 361. 2 k. J H = -1451. 1 k. J Cancel terms and take sum. 4 NH 3 + 5 O 2 4 NO + 6 H 2 O H = -906. 3 k. J Is the reaction endothermic or exothermic? 4
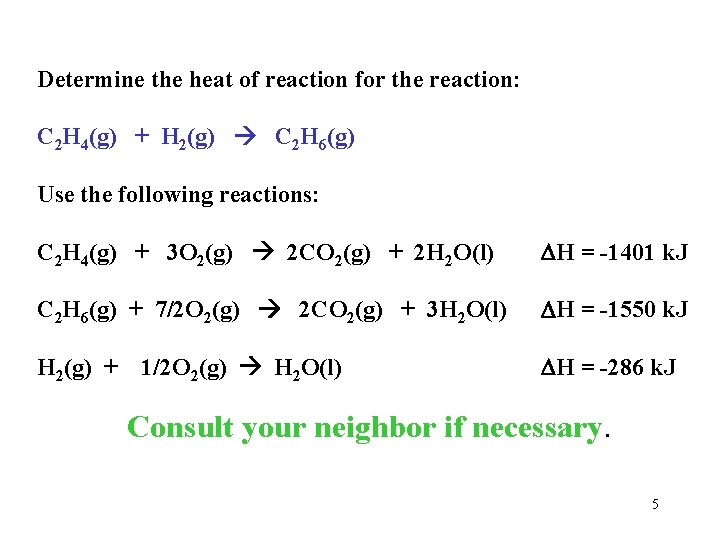
Determine the heat of reaction for the reaction: C 2 H 4(g) + H 2(g) C 2 H 6(g) Use the following reactions: C 2 H 4(g) + 3 O 2(g) 2 CO 2(g) + 2 H 2 O(l) H = -1401 k. J C 2 H 6(g) + 7/2 O 2(g) 2 CO 2(g) + 3 H 2 O(l) H = -1550 k. J H 2(g) + 1/2 O 2(g) H 2 O(l) H = -286 k. J Consult your neighbor if necessary. 5

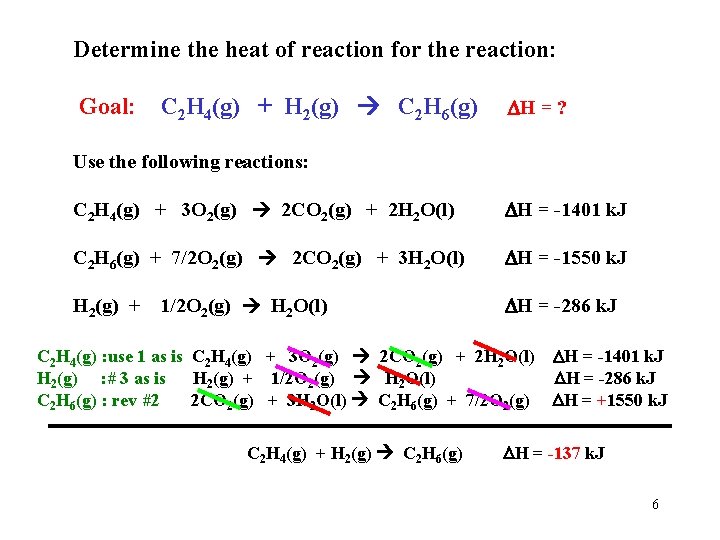
Determine the heat of reaction for the reaction: Goal: C 2 H 4(g) + H 2(g) C 2 H 6(g) H = ? Use the following reactions: C 2 H 4(g) + 3 O 2(g) 2 CO 2(g) + 2 H 2 O(l) H = -1401 k. J C 2 H 6(g) + 7/2 O 2(g) 2 CO 2(g) + 3 H 2 O(l) H = -1550 k. J H 2(g) + 1/2 O 2(g) H 2 O(l) H = -286 k. J C 2 H 4(g) : use 1 as is C 2 H 4(g) + 3 O 2(g) 2 CO 2(g) + 2 H 2 O(l) H = -1401 k. J H 2(g) : # 3 as is H 2(g) + 1/2 O 2(g) H 2 O(l) H = -286 k. J C 2 H 6(g) : rev #2 2 CO 2(g) + 3 H 2 O(l) C 2 H 6(g) + 7/2 O 2(g) H = +1550 k. J C 2 H 4(g) + H 2(g) C 2 H 6(g) H = -137 k. J 6

Summary: enthalpy is a state function and is path independent. 7

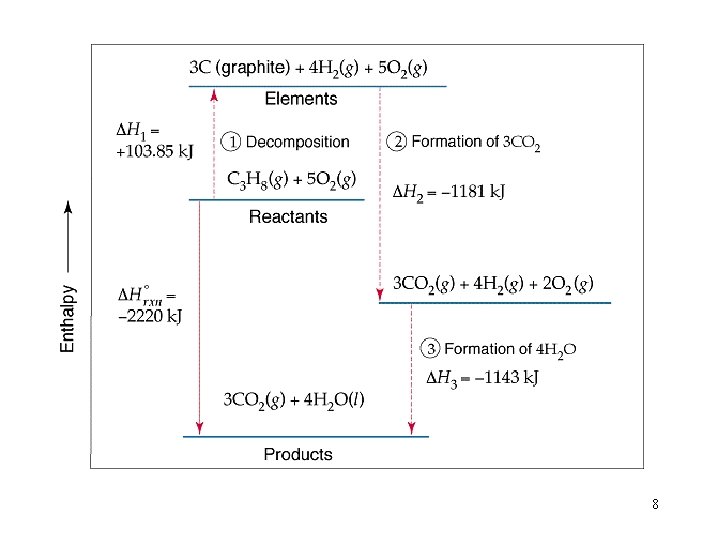
8

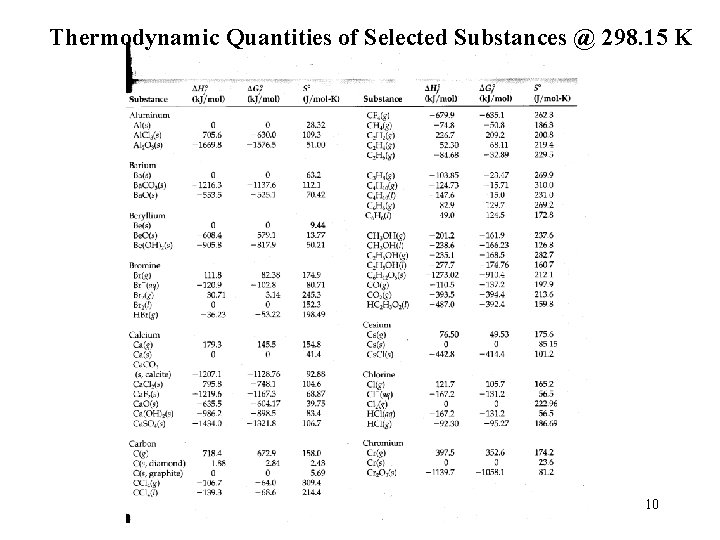
Standard Enthalpies of formation: 9

Thermodynamic Quantities of Selected Substances @ 298. 15 K 10