Hess Law and Enthalpy of Formation State Function









- Slides: 14

Hess’ Law and Enthalpy of Formation

State Function revisited…. Dependent ONLY on a system’s state at a given moment in time. Only initial and final states Not based on the path to get to a given condition Ex. Energy, Enthalpy

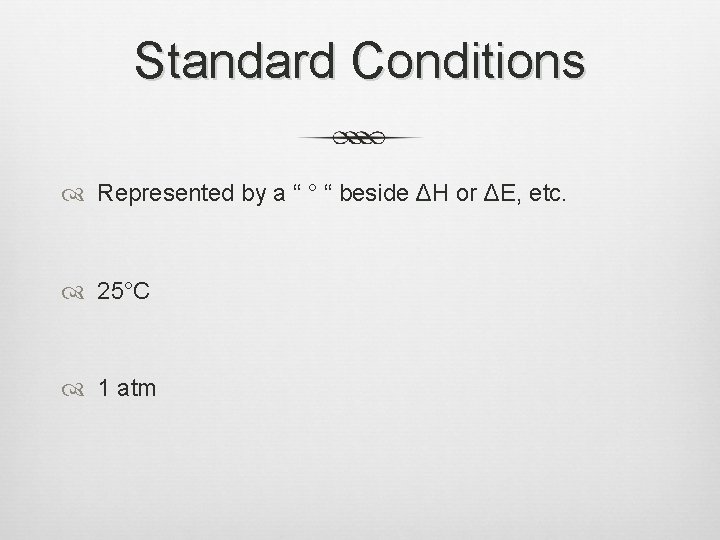
Standard Conditions Represented by a “ ° “ beside ΔH or ΔE, etc. 25°C 1 atm

Methods for determining ΔH 1) Calorimetry 2) Application of Hess’ Law

Hess’ Law Enthalpy change for a chemical reaction is the same whether it occurs in multiple steps or one step ΔHrxn = ΣΔHA+B+C (sum of ΔH for each step) Allows us to break a chemical reaction down into multiple steps to calculate ΔH Add the enthalpies of the steps for the enthalpy for the overall chemical reaction

Guidelines for using Hess’ Law Must use data and combine each step in a way that gives the chemical reaction with the unknown ΔH Set up steps so chemical compounds not in the final reaction are cancelled Reverse a reaction if necessary and change the sign on ΔH Check for correct mole ratios

Example 1: H 2 O(l) H 2 O (g) ΔH° = ? Based on the following: H 2 + ½ O 2 H 2 O(l) ΔH° = -285. 83 k. J/mol H 2 + ½ O 2 H 2 O(g) ΔH° = -241. 82 k. J/mol

Example 2: C(s) + 4 H 2 C 3 H 8 (g) ΔH° = ? Based on the following: 2 H 2 + O 2 2 H 2 O ΔH° = -571. 7 k. J/mol C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O(g) ΔH°= -2220. 1 k. J/mol C(s) + O 2 CO 2 (g) ΔH° = -393. 5 k. J/mol

Methods for determining ΔH 1) Calorimetry 2) Application of Hess’ Law 3) Enthalpies of Formation

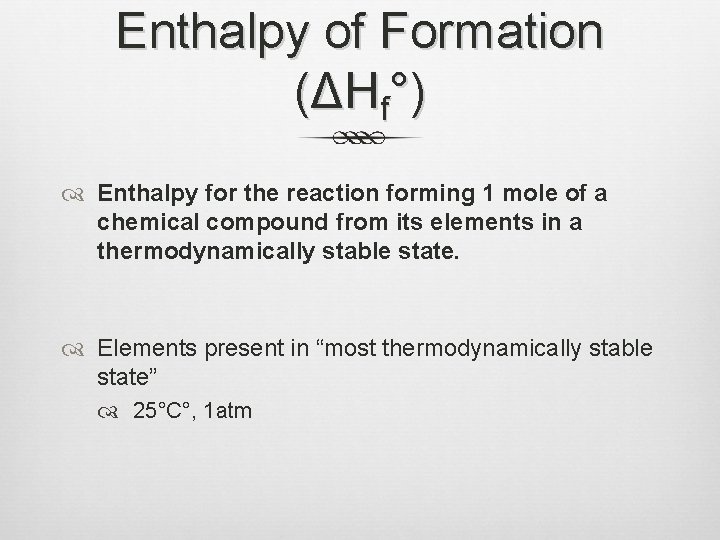
Enthalpy of Formation (ΔHf°) Enthalpy for the reaction forming 1 mole of a chemical compound from its elements in a thermodynamically stable state. Elements present in “most thermodynamically stable state” 25°C°, 1 atm

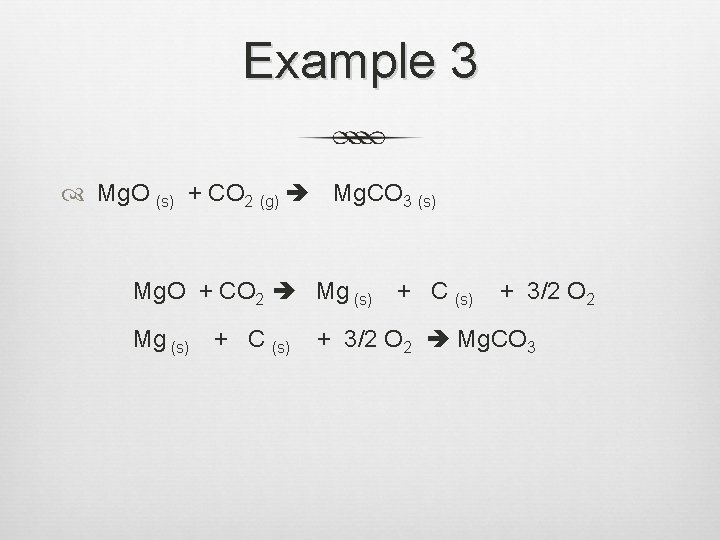
Example 3 Mg. O (s) + CO 2 (g) Mg. CO 3 (s) Mg. O + CO 2 Mg (s) + C (s) + 3/2 O 2 Mg. CO 3

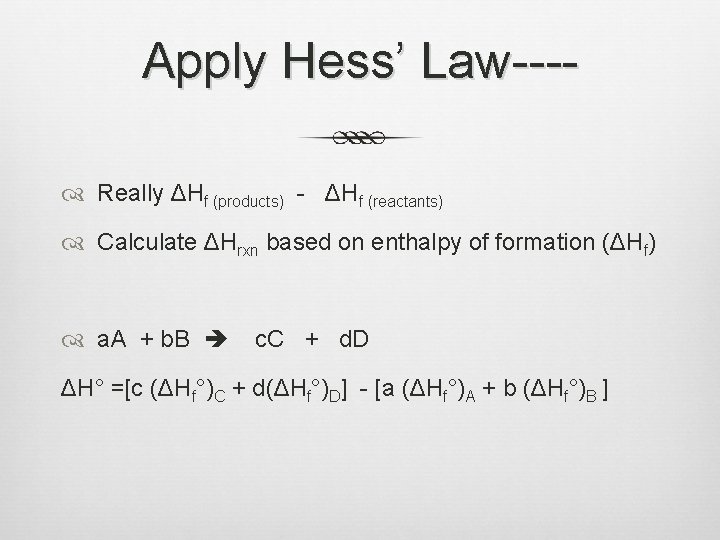
Apply Hess’ Law--- Really ΔHf (products) - ΔHf (reactants) Calculate ΔHrxn based on enthalpy of formation (ΔHf) a. A + b. B c. C + d. D ΔH° =[c (ΔHf°)C + d(ΔHf°)D] - [a (ΔHf°)A + b (ΔHf°)B ]

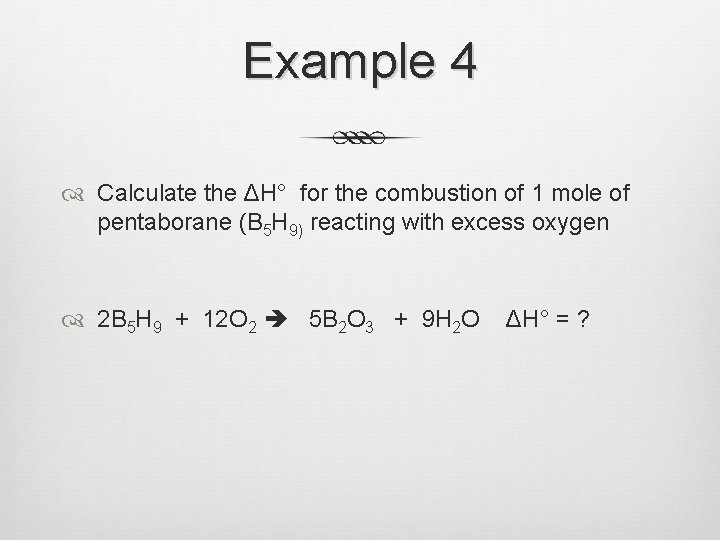
Example 4 Calculate the ΔH° for the combustion of 1 mole of pentaborane (B 5 H 9) reacting with excess oxygen 2 B 5 H 9 + 12 O 2 5 B 2 O 3 + 9 H 2 O ΔH° = ?

Example 5 Isopropyl alcohol (rubbing alcohol) undergoes a combustion reaction 2(CH 3)2 CHOH + 9 O 2 6 CO 2 + 8 H 2 O ΔH° = -4011 k. J/mol Calculate the standard enthalpy of formation for isopropyl alcohol.