Herpesviridae INTRODUCTION TO THE FAMILY HERPESVIRIDAE GENERAL CHARACTERISTICS



































- Slides: 52

Herpesviridae

INTRODUCTION TO THE FAMILY HERPESVIRIDAE GENERAL CHARACTERISTICS ØHerpesviruses are amongst the largest human viruses ØHV infections have been recognised since ancient times ØHerpesviruses are highly disseminated in nature ØTo date, there are 8 known human Herpesviruses.

Subfamily Biological properties Alphaherpesvirinae ØVariable host range ØRapid reproductive cycle ØEstablish latent infections in neurons Herpes simplex virus type 1 Ø (HSV-1) Herpes simplex virus type 2 Ø (HSV-2) Varicella-Zoster virus (VZV) Ø HHV-3 Betaherpesvirinae Generally restricted host rangeØ Slow reproductive cycleØ CytomegalicØ Establish latency in lymphocytes, Ø monocytes, SG, kidney. Human cytomegalovirus Ø (HCMV) HHV-5 Human herpesvirus-6 (HHV-Ø 6) Human herpesvirus-7 (HHV-Ø 7) ØRestricted host range ØLymphotropic, lymphproliferative. ØEstablish latent infections in lymphoid cells ØEpstein-Barr virus (EBVHHV-4) ØHuman herpesvirus-8 (HHV 8) α β Gammaherpesvirinae γ

Betaherpesvirinae ØHuman cytomegalovirus (HCMV) HHV-5 ØHuman herpesvirus-6 (HHV-6) ØHuman herpesvirus-7 (HHV-7)

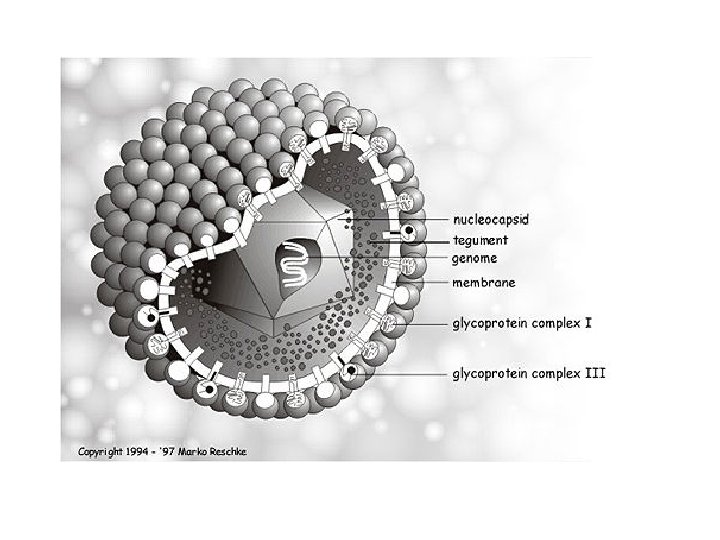
• A member of the herpesvirus family • ds DNA enveloped virus • 2 additional forms are seen in cell culture ; (i) "dense body", which does not contain DNA or nucleocapsid (ii) "non-infectious enveloped particle" (NIEP), consists of an empty capsid surrounded by a lipid envelope.


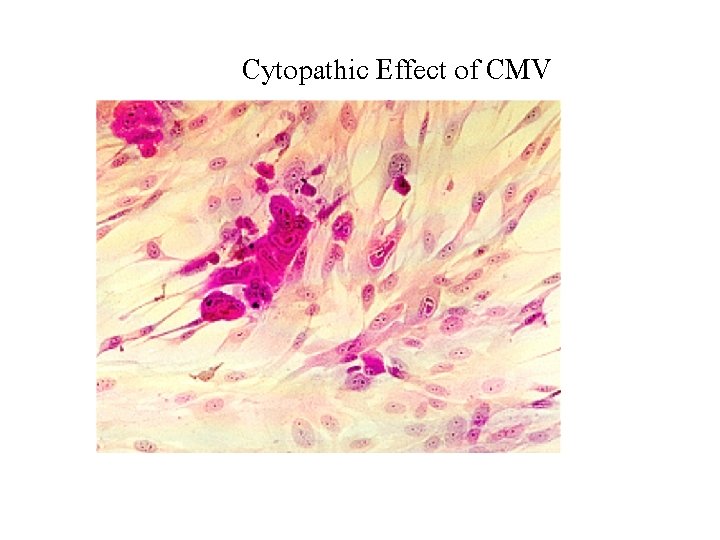
Cytomegalovirus • CMV is a cytotoxic virus that alters the metabolic biochemical cascade of cells, resulting in cell enlargement (cytomegaly) and condensation of nuclear mass (nuclear inclusion). • Infection in most organs kidney, liver, intestine, lung, brain, salivary gland • chronic infection latent infection; white blood cells

Hematoxylin-eosin–stained lung section showing typical owl-eye inclusions

CMV Transmission * (Vertical) In utero: Following primary(40– 50%) or recurrent (1%) CMV infection in a pregnant woman * Perinatally genital secretion, breast milk 2 -10% infants infected within 6 months * Postnatally Saliva, sexual transmission, blood and transplanted organs

Potential sources of virus • Saliva • Breast milk • Blood • Urine • Semen/cervical secretion • Transplanted organs

Epidemiology • In developed countries with a high standard of hygiene, 40% of adolescents are infected and ultimately 70% of the population is infected. • In developing countries, over 90% of people are ultimately infected.

Congenital Infection • Defined as the isolation of CMV from the saliva or urine within 3 weeks of birth. • Commonest congenital viral infection, affects 0. 3 - 1% of all live births. • The 2 nd most common cause of mental handicap (more cases of congenital damage than rubella. ) • May be transmitted to the foetus during all stages of pregnancy.

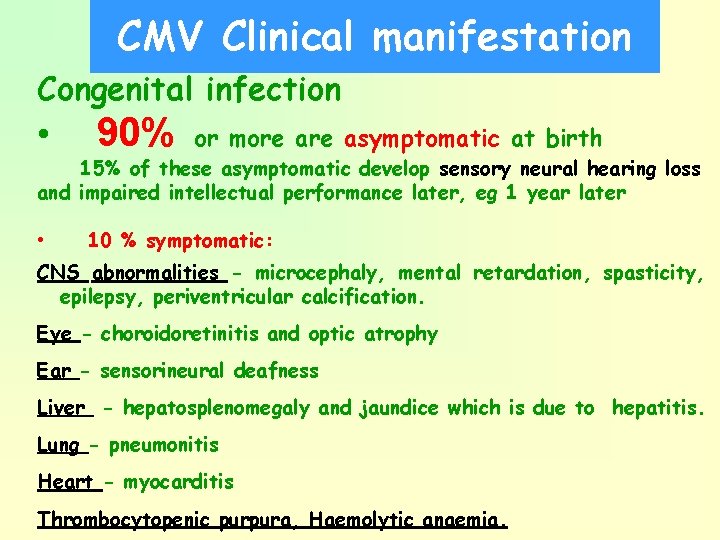
CMV Clinical manifestation Congenital infection • 90% or more asymptomatic at birth 15% of these asymptomatic develop sensory neural hearing loss and impaired intellectual performance later, eg 1 year later • 10 % symptomatic: CNS abnormalities - microcephaly, mental retardation, spasticity, epilepsy, periventricular calcification. Eye - choroidoretinitis and optic atrophy Ear - sensorineural deafness Liver - hepatosplenomegaly and jaundice which is due to hepatitis. Lung - pneumonitis Heart - myocarditis Thrombocytopenic purpura, Haemolytic anaemia.

To. RCH screening

Perinatally • Asymptomatic • CMV pneumonitis.

Post natal infection • Infection in normal host • <90 %are subclinical • Clinical -> mononucleosis- like syndrome( young adults(

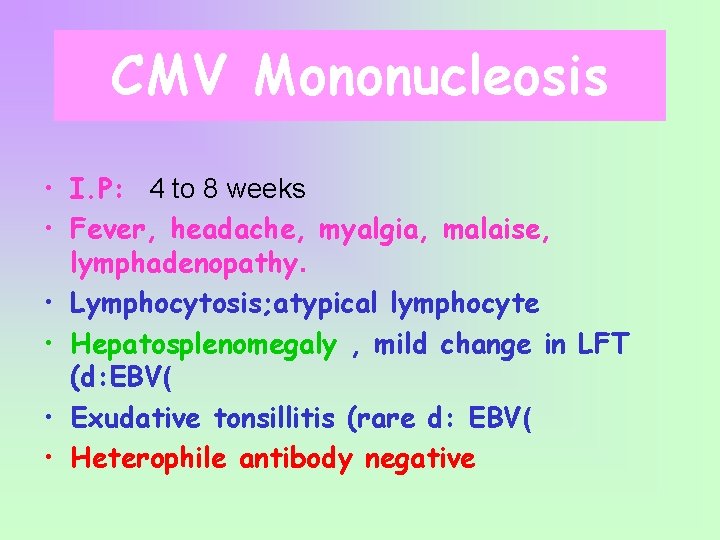
CMV Mononucleosis • I. P: 4 to 8 weeks • Fever, headache, myalgia, malaise, lymphadenopathy. • Lymphocytosis; atypical lymphocyte • Hepatosplenomegaly , mild change in LFT (d: EBV( • Exudative tonsillitis (rare d: EBV( • Heterophile antibody negative

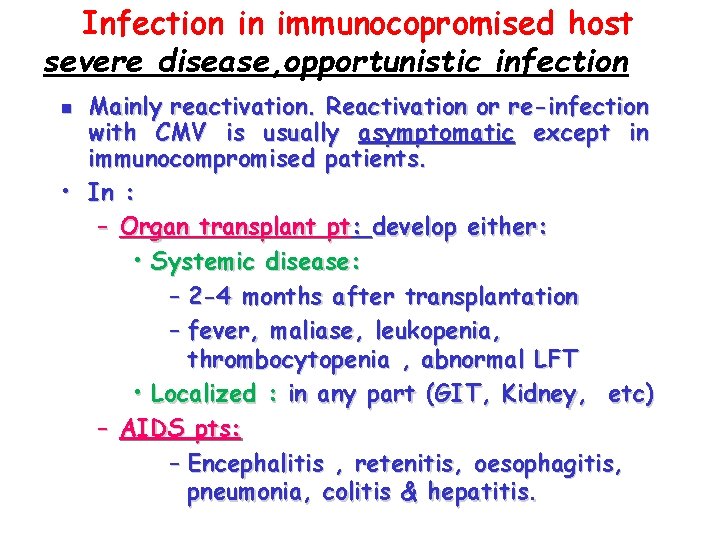
Infection in immunocopromised host severe disease, opportunistic infection Mainly reactivation. Reactivation or re-infection with CMV is usually asymptomatic except in immunocompromised patients. • In : – Organ transplant pt: develop either: • Systemic disease: – 2 -4 months after transplantation – fever, maliase, leukopenia, thrombocytopenia , abnormal LFT • Localized : in any part (GIT, Kidney, etc) – AIDS pts: – Encephalitis , retenitis, oesophagitis, pneumonia, colitis & hepatitis. n

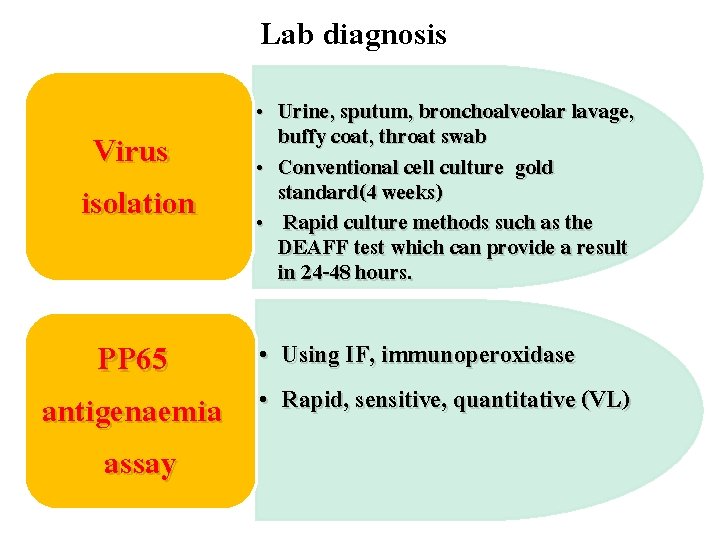
Lab diagnosis Virus isolation PP 65 antigenaemia assay • Urine, sputum, bronchoalveolar lavage, buffy coat, throat swab • Conventional cell culture gold standard(4 weeks) • Rapid culture methods such as the DEAFF test which can provide a result in 24 -48 hours. • Using IF, immunoperoxidase • Rapid, sensitive, quantitative (VL)

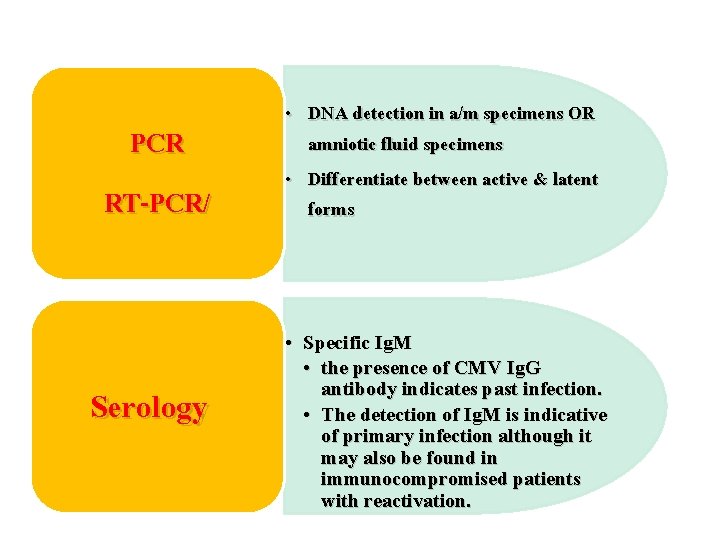
PCR RT-PCR/ Serology • DNA detection in a/m specimens OR amniotic fluid specimens • Differentiate between active & latent forms • Specific Ig. M • the presence of CMV Ig. G antibody indicates past infection. • The detection of Ig. M is indicative of primary infection although it may also be found in immunocompromised patients with reactivation.

CMV pp 65 antigenaemia test

DEAFF test for CMV

Cytopathic Effect of CMV

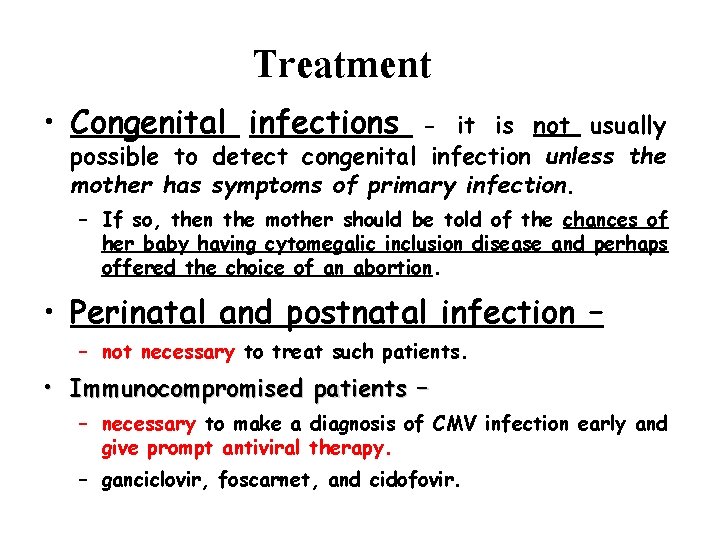
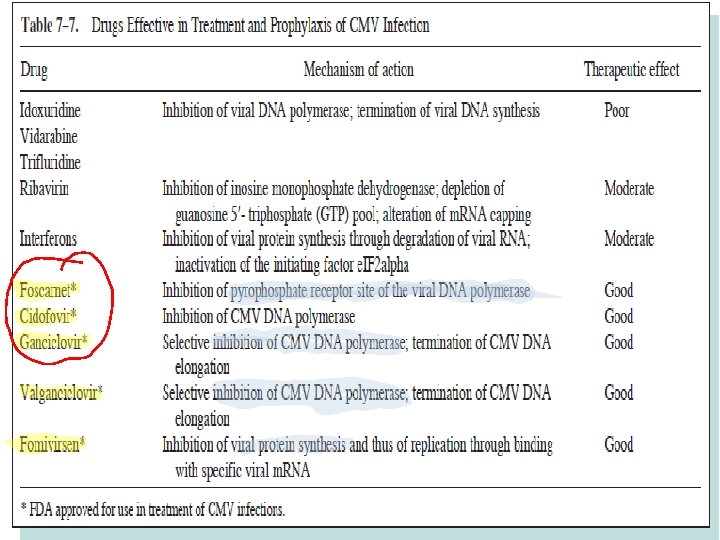
Treatment • Congenital infections - it is not usually possible to detect congenital infection unless the mother has symptoms of primary infection. – If so, then the mother should be told of the chances of her baby having cytomegalic inclusion disease and perhaps offered the choice of an abortion. • Perinatal and postnatal infection – – not necessary to treat such patients. • Immunocompromised patients – – necessary to make a diagnosis of CMV infection early and give prompt antiviral therapy. – ganciclovir, foscarnet, and cidofovir.


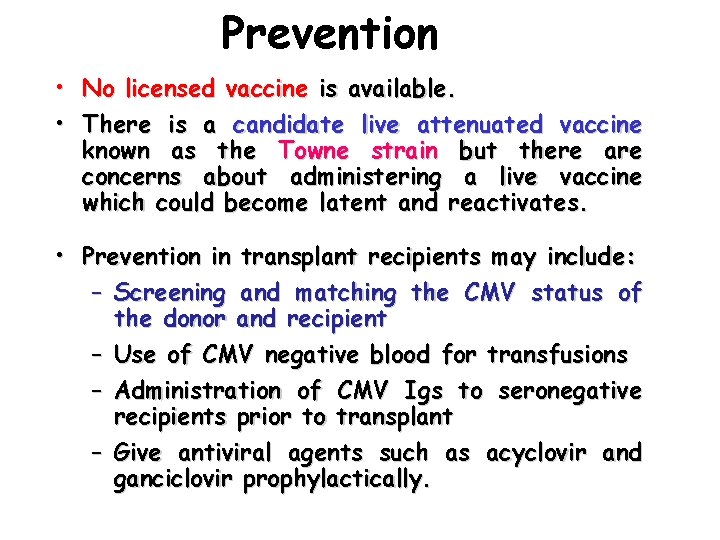
Prevention • No licensed vaccine is available. • There is a candidate live attenuated vaccine known as the Towne strain but there are concerns about administering a live vaccine which could become latent and reactivates. • Prevention in transplant recipients may include: – Screening and matching the CMV status of the donor and recipient – Use of CMV negative blood for transfusions – Administration of CMV Igs to seronegative recipients prior to transplant – Give antiviral agents such as acyclovir and ganciclovir prophylactically.

Human herpesvirus 6

Human herpesvirus 6 • Two variants: A and B (B common in symptomatic infection) • First isolated from B-lymphocyte in 1986 -> Human B lymphotropic virus • Target cells; T-lymphocyte B-lymphocyte, monocyte/macrophage, epithelial cell • Latent infection; monocyte/macrophage, salivary gland

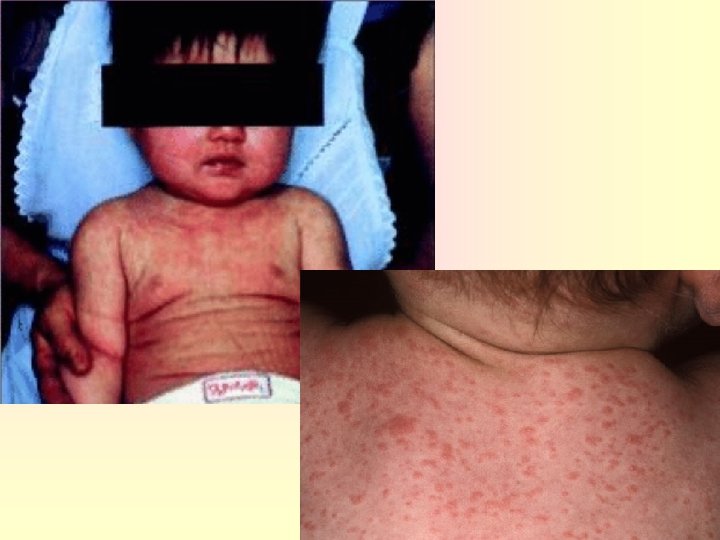
HHV-6 Clinical Manifestation Exanthem subitum (roseola infantum( 6 th disease • Infection can be inapparent, or mild. • By school age , nearly 100%of children are infected. • common in infants age 6 m- 3 yr. • Abrupt onset of high grade fever • skin rash, • 3 -5 days later • non pruritic, maculopapular , starts at face, trunk then other parts • Disappears 1 -3 days later without desquamation or pigment change


Human herpesvirus 6 * 1 ry infection is rare in adults, if it occurs →Mononucleosis-like syndrome, hepatitis, prolonged lymphadenopathy • Reactivation is commonly asymptomatic(virus excreted without clinical symptoms).

Human herpesvirus 6 Immunocompromised patients • • Fever Hepatitis Lung infection In AIDS pts…… disease progression.

Human herpesvirus 6 Complication • • • FEBRILE CONVULSIONS. Aseptic meningitis Meningoencephalitis Encephalitis. Mononucleosis like syndrome. Bone marrow suppression Rash without fever Hepatitis Lung infection

Human herpesvirus 6 Lab diagnosis • Virus isolation: – From – peripheral monouclear cells – Salive – Conventional shell vial culture. • Ag detection: – IF • PCR: – For – peripheral blood monocytes – CSF – Saliva • serology: Ig. M (Ig. G are present)

Human herpesvirus 7 • HHV-6 and HHV-7 share limited nucleotide homology and antigenic cross-reactivity. • 75% of adults have HHV-7 in their saliva • First isolated from CD 4+ T cells from healthy person in 1990 • Need CD 4 receptor for infection • Infection acquired after 2 nd yr. • Virus secreted in saliva • Clinical manifestation; exanthem subitum(but later than HHV 6), common cold

Human herpesvirus 4 (Epstein-Barr Virus ; EBV(

EBV Infection # Wide spectrum of diseases subclinical -- > severe disease # Site of replication oropharynx, salivary gl. , B-lymphocyte # Lifelong carrier state: chronic shedding # Latent infection throat, lymphoid tissue, B-lymphocyte

EBV Epidemiology Ubiquitous: early adulthood The prevalence of infection 95% by Developed countries; preschool aged 2 peaks 1 -6 years 14 -20 years (80 - adults aged Developing countries; 90% ) 90% get infection within the first Infection in early life : asymptomatic If at adolescence : 50 % develop infectious mononucleosis ( usual age 17 -25 years) 2 years of lif


EBV Transmission • Saliva through kissing • Blood transfusion/contact • cervical secretion • semen

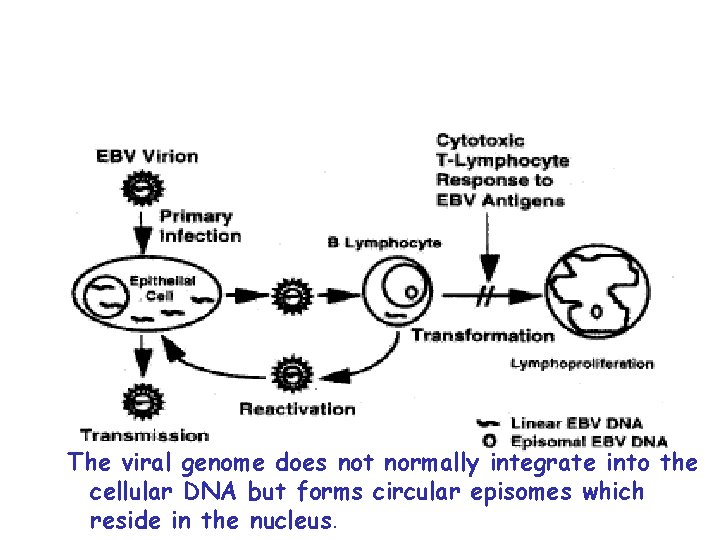
Pathogenesis • Once infected, a lifelong carrier state develops whereby a low grade infection is kept in check by the immune defenses. • Low grade virus replication and shedding can be demonstrated in the epithelial cells of the pharynx of all seropositive individuals. • EBV is able to immortalize B-lymphocytes in vitro and in vivo

The viral genome does not normally integrate into the cellular DNA but forms circular episomes which reside in the nucleus.

EBV Mononucleosis glandular fever Incubation period 30 -50 days • prodrome 2 – 5 day: Fever, maliase, headache, myalgias • acute phase - fever (last 4– 5 wks), Lymphadenopathy (cervical or generalized) (2– 4 wks) – exudative or nonexudative pharyngitis/tonsillitis (massive( – Lymphocytosis with atypical lymphocyte – splenomegaly ( sometimes e hepatomegaly→hepatitis( – Heterophile antibody positive. – eyelid oedema 15% – Skin rash • resolution phase : organomegaly may persist 1– 3 m

IM Treatment Medical Care : u self-limited illness : not require specific therapy. u Inpatient therapy of medical and surgical complications may be required. u Acyclovir (10 mg/kg/dose IV q 8 h for 7 -10 d) – inhibit viral shedding from the oropharynx u IVIG (400 mg/kg/d IV for 2 -5 d) – immune thrombocytopenia associated with Andersson J et al. J Infect Dis. Feb 1986; 153(2): 283 -90. Cyran EM et al. Am J Hematol. Oct 1991; 38(2): 124 -9.

IM : Prevention u u u Isolation is not required : low transmission. Avoid contact with saliva. Do not kiss children on the mouth. Maintain clean conditions : day care, avoid sharing toys. EBV can be transmitted by blood transfusion and by bone marrow transplantation. Vaccine development is proceeding, although the role of a vaccine is unclear. FEIGIN et al. Textbook of Pediatric Infectious Diseases 5 th ed; 2004: 1952 -1957.

Infectious Mononucleosis : Lab u The 3 classic criteria for laboratory confirmation 1 lymphocytosis 2 the presence of at least 10% atypical lymphocytes on peripheral smear 3 a positive serologic test ) EBV. (

Lab diagnosis CBC Nonspesefic serology • Lymphocytosis , atypical lymphocytes • Heterophile antibody (Paul Bunnel) • Ig. M • More than 90% adults, 80% of children • diagnostic • Detected by: Paul Bunnel (old method) • Monospot (slide agglutination) • Heterophile negative mono =mononuc – like syndrome: Toxo/ CMV/ HIV

Specific serology • If infection is suspected, and Monospot is negative, test for specific antibodies: – VCA: in acute infection, VCA Ig. M is positive. Diagnostic. VCA Ig. G is positive. Not diagnostic(coz, persists for 1 year) - EA : 2 forms - (D)Diffuse: detected in cytoplasm and nucleus of infected cells. Positive in infectious mononucleoses. - (R)Restrected : to cytoplasm. Negative in Infectious mono. Develops later on. - EBNA: negative in infectious mono. And appears later , persists for life.

Infectious Mononucleosis atypical lymphocytes : Downey types

Human herpesvirus 8 *Originally isolated from cell of Kaposi’s sarcoma (KS( * HHV-8 DNA found in almost 100% in KS * Not ubiquitous infection low seroprevalence in general population

Human herpesvirus 8 Diseases associated with HH 8: • AIDS associated Kaposi sarcoma. • Castelman Disease • Body cavity lymphoma.

Human herpesvirus 8 Diagnosis: • Virus isolation. • PCR detection of virus in tissues.