Hereditary diseases Congenital malformations Dr Gyrgy Fekete Congenital





















- Slides: 89

Hereditary diseases. Congenital malformations. Dr. György Fekete

Congenital malformations • • • Conception – organogenesis - birth Genetic causes Environmental factors (teratogens) Visible/ recognazible at birth Later manifestation

• Genetic conditions: causes of acute and chronic diseases • Onset of disease: fetus, infant, child, adult • Genetic abnormalities may produce: congenital malformations, metabolic disturbances, specific organ dysfunction, abnormalities of sexual differentiation

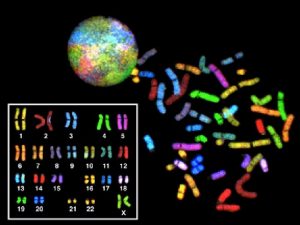
• Monogenic diseases: 1% of newborn babies • Chromosomal aberrations: 0. 5% • Multifactorial disorders: 1 -3%

• Monogenic diseases: 1% of newborn babies • Chromosomal aberrations: 0. 5% • Multifactorial disorders: 1 -3%

Inherited conditions • If a single allele has a detectable effect: dominant • If two functionally identical alleles cause the effect: recessive • XY males are hemizygous for genes on the X chromosome

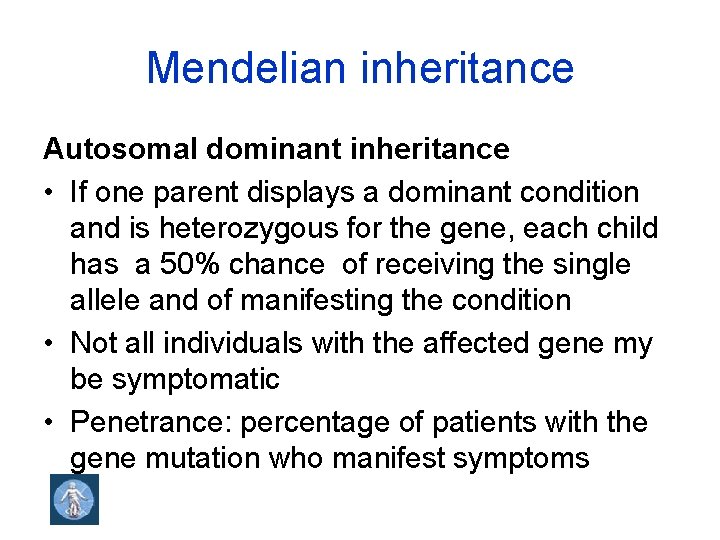
Mendelian inheritance Autosomal dominant inheritance • If one parent displays a dominant condition and is heterozygous for the gene, each child has a 50% chance of receiving the single allele and of manifesting the condition • Not all individuals with the affected gene my be symptomatic • Penetrance: percentage of patients with the gene mutation who manifest symptoms

• Expressivity: spectrum of severity in patiens having clinical manifestation • Examples: achondroplasia, Crouzon syndrome, neurofibromatosis type I, II, Marfan syndrome, hereditary angioneurotic edema (HANE)

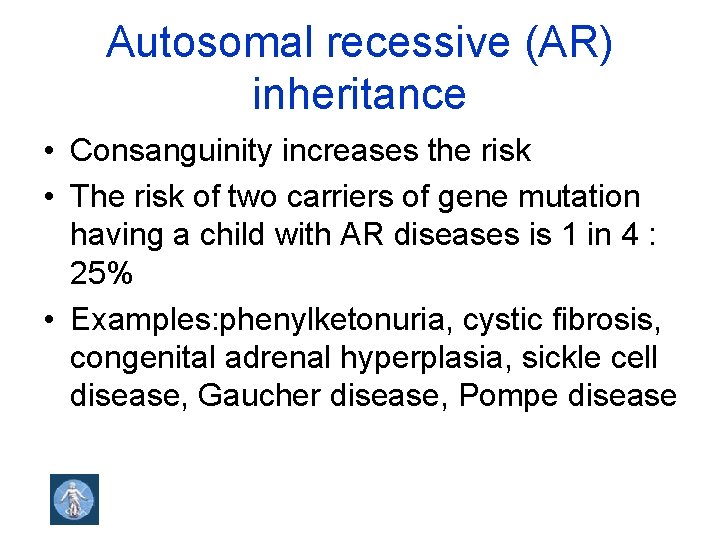
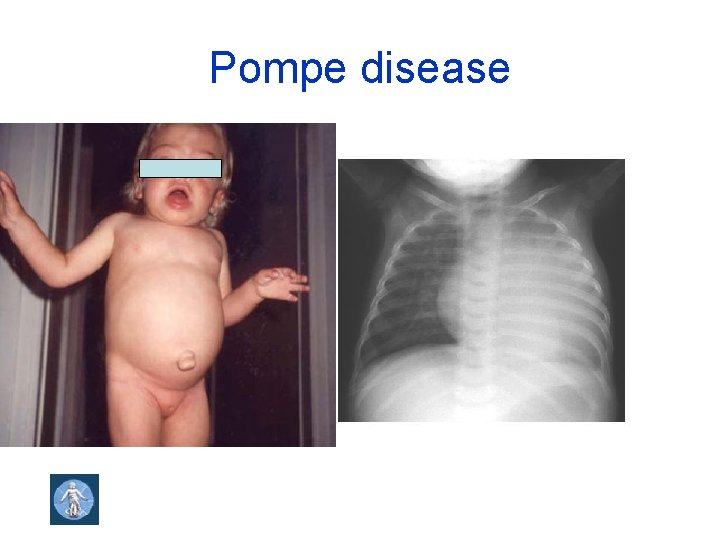
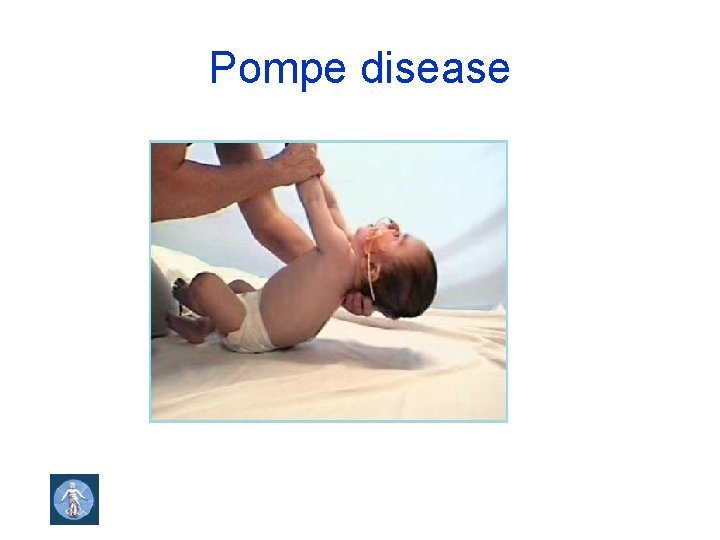
Autosomal recessive (AR) inheritance • Consanguinity increases the risk • The risk of two carriers of gene mutation having a child with AR diseases is 1 in 4 : 25% • Examples: phenylketonuria, cystic fibrosis, congenital adrenal hyperplasia, sickle cell disease, Gaucher disease, Pompe disease

Sex – linked inheritance • The gene locus is on the X chromosome • When the mother is a carrier of the gene mutation, 50% of male offspring will have the disease, and 50% of female offspring will be carriers • All daughters of the ill father will be obligate carriers • Examples: Duchenne muscular dystrophy (DMD), hemophilia A and B, adrenoleukodystrophy, Fabry disease

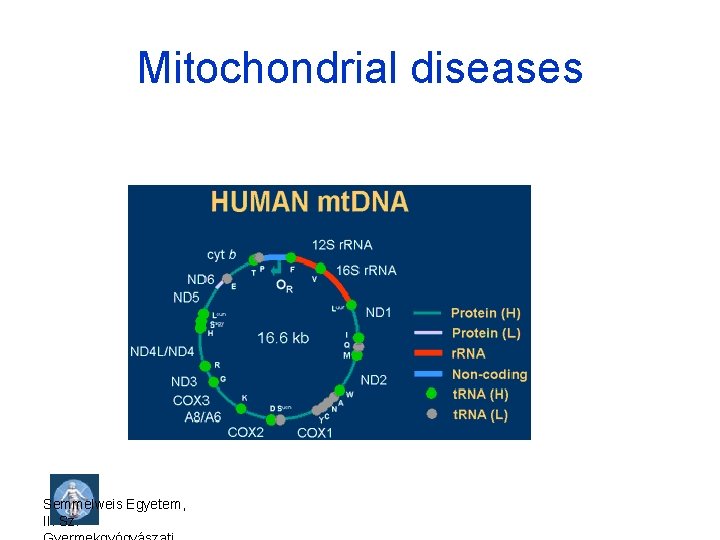
Mitochondrial diseases Semmelweis Egyetem, II. Sz.

Importance • Prevalence in newborns: 46 -50 %o • 25 -40 per cent of infant mortality • One factor in prematurity and intrauterine dystrophy • Severe conditions • Burden: child, family, society

Classification • Severity: major and minor malformations • Major: hindering significant organ functions – Isolated (GI atresias, Fallot – tetralogy) – Multiple: two or more organs, organ systems – Genetic: chromosome aberration, monolocus, other mechanism (uniparental disomy, genomic imprinting, triplet expansion, mitochondrial) – Teratogens (TORCH, chemicals, drugs, irradiation) – Genetic + environmental (multifactorial, complex diseases)

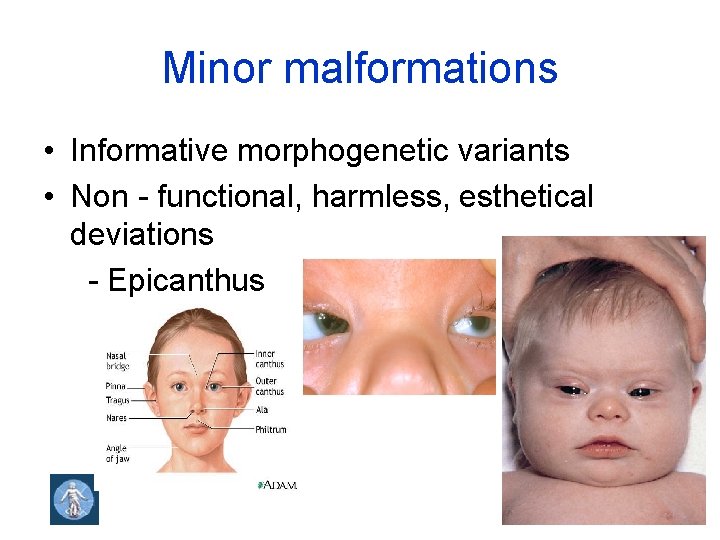
Minor malformations • Informative morphogenetic variants • Non - functional, harmless, esthetical deviations - Epicanthus

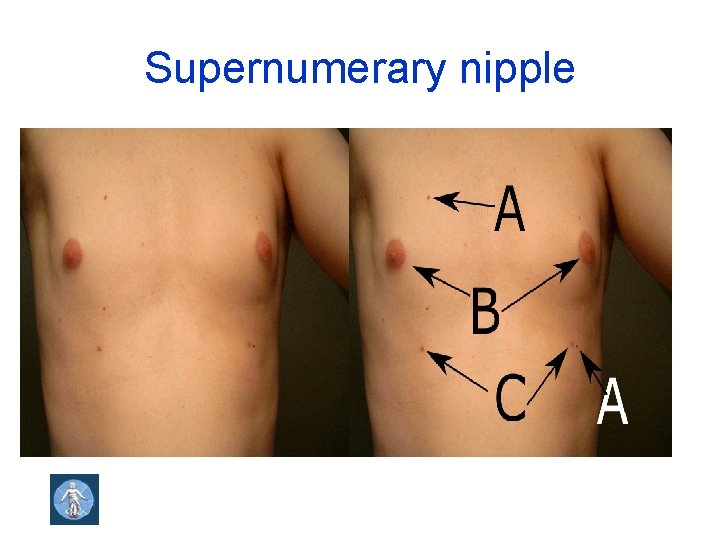
Supernumerary nipple

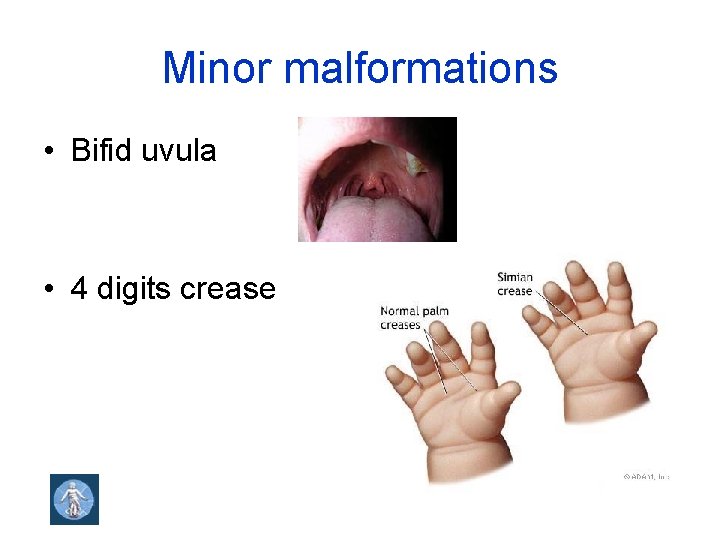
Minor malformations • Bifid uvula • 4 digits crease

Hypertelorism

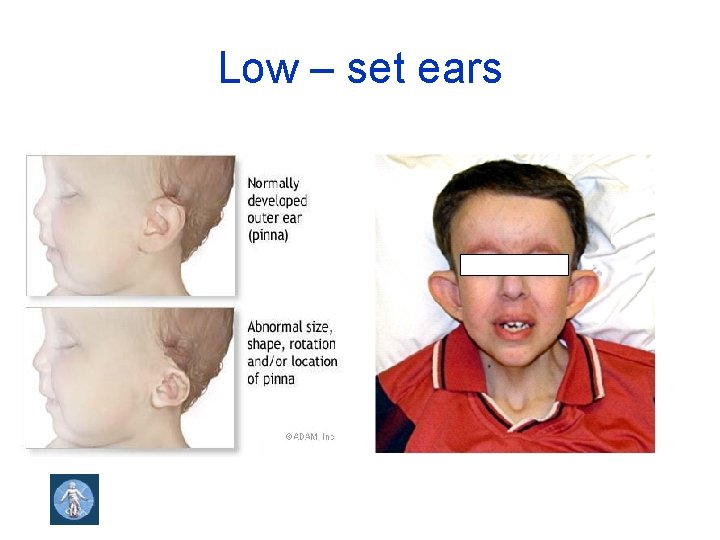
Low – set ears

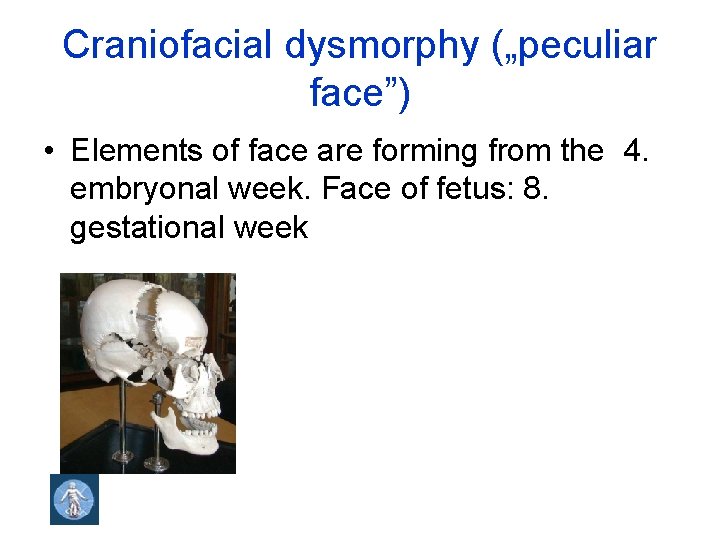
Craniofacial dysmorphy („peculiar face”) • Elements of face are forming from the 4. embryonal week. Face of fetus: 8. gestational week

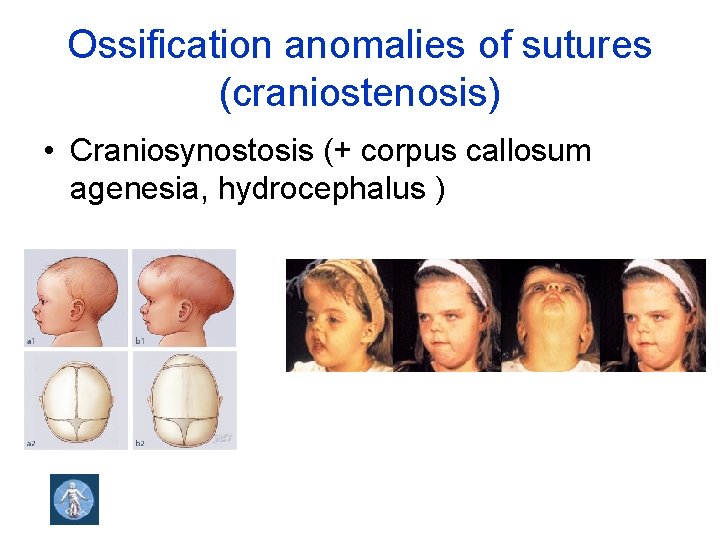
Ossification anomalies of sutures (craniostenosis) • Craniosynostosis (+ corpus callosum agenesia, hydrocephalus )

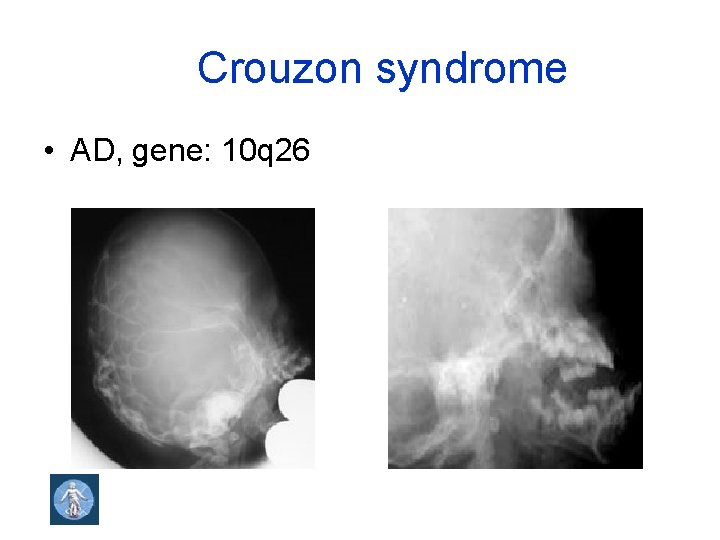
Crouzon syndrome • AD, gene: 10 q 26

• Crouzon syndrome • Apert syndrome (Acrocephalosyndactyly type I) • Gene: 10 q 26, fibroblast growth factor receptor-2 (FGFR-2) • Advanced paternal age ( > 45 yrs) • Pfeiffer syndrome (Acrocephalosyndactyly type V) • Gene: 10 q 26, 8 p 11. 2 -11. 1(FGFR-1)

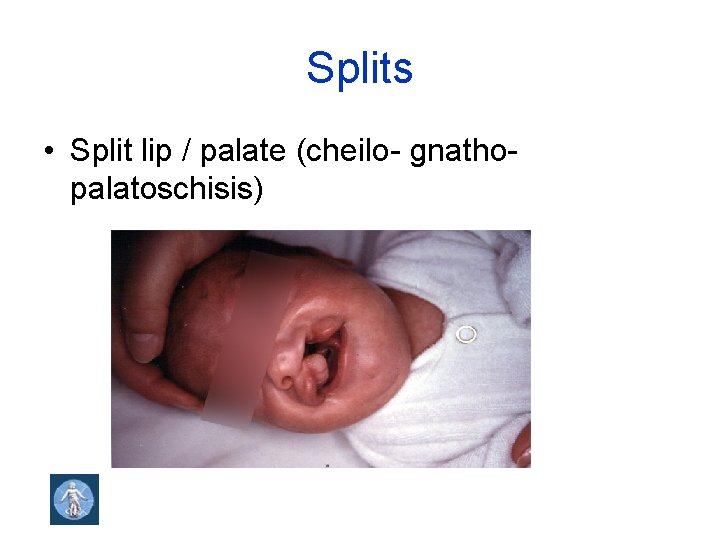
Splits • Split lip / palate (cheilo- gnatho- palatoschisis)

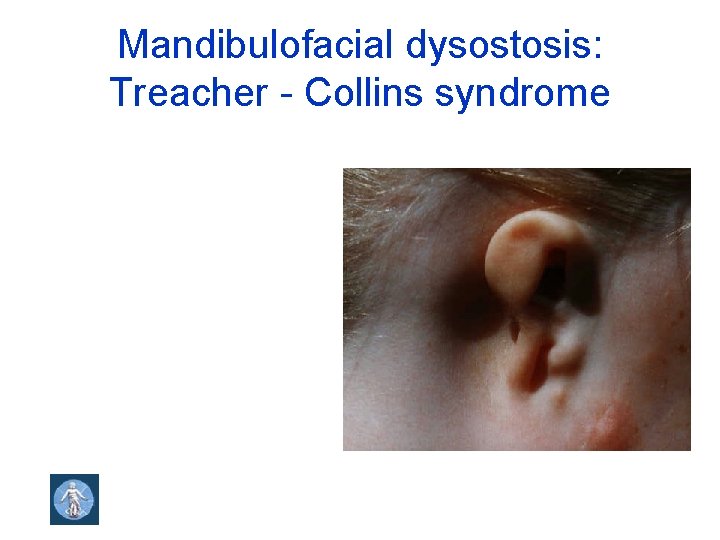
Mandibulofacial dysostosis: Treacher - Collins syndrome

Steps of examination • Parents, sibs, grandparents: resemblance? (photos!) • Anatomical/ morphological deviation? • Isolated or multiple? • Psychomotor retardation? • Other minor malformations? Hidden malformations (internal organs) ? • Teratogenic exposition? • Special methods / investigations • Councelling: prognosis, therapy

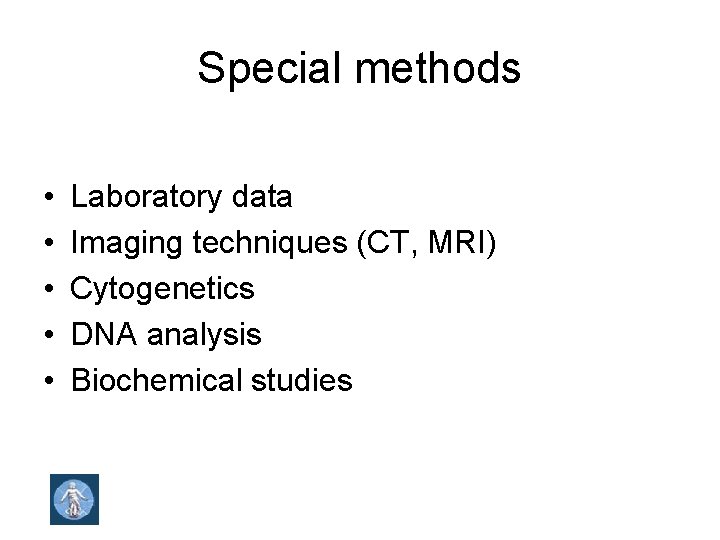
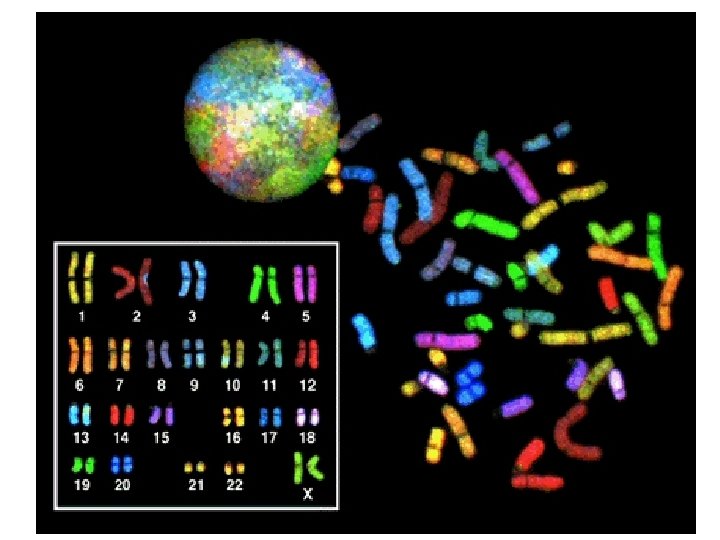
Special methods • • • Laboratory data Imaging techniques (CT, MRI) Cytogenetics DNA analysis Biochemical studies

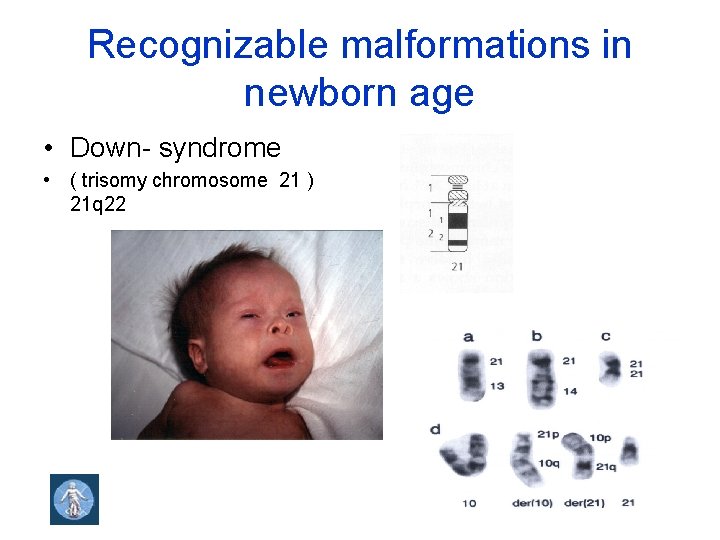
Recognizable malformations in newborn age • Down- syndrome • ( trisomy chromosome 21 ) 21 q 22

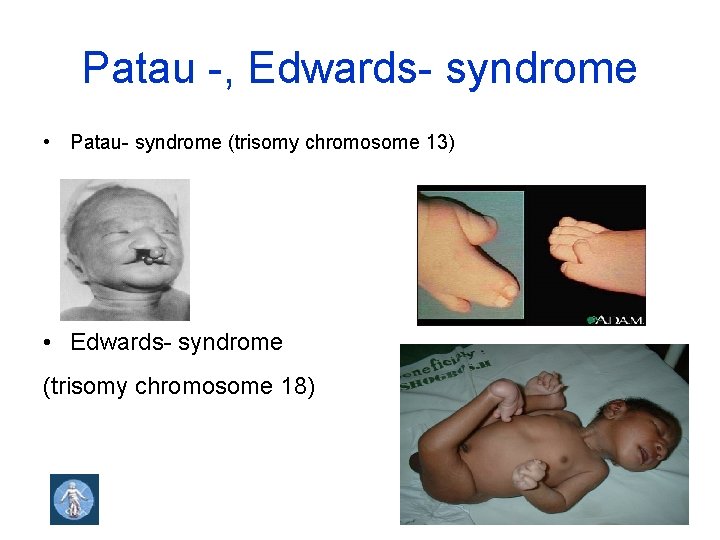
Patau -, Edwards- syndrome • Patau- syndrome (trisomy chromosome 13) • Edwards- syndrome (trisomy chromosome 18)

Prader – Willi syndrome

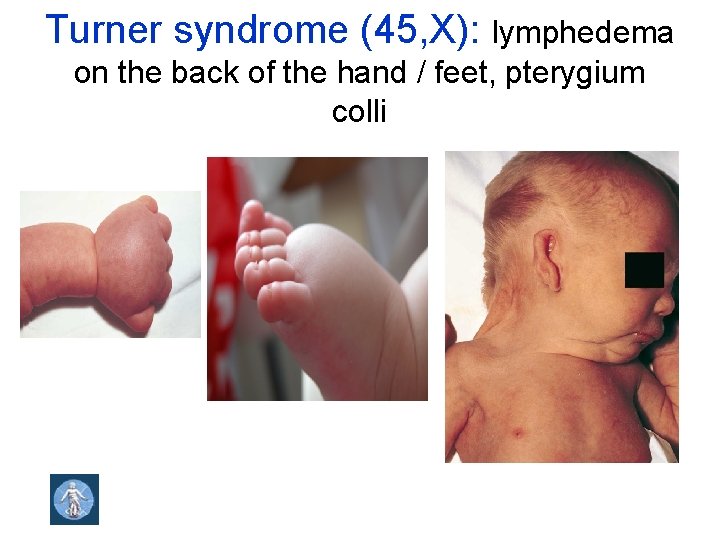
Turner syndrome (45, X): lymphedema on the back of the hand / feet, pterygium colli


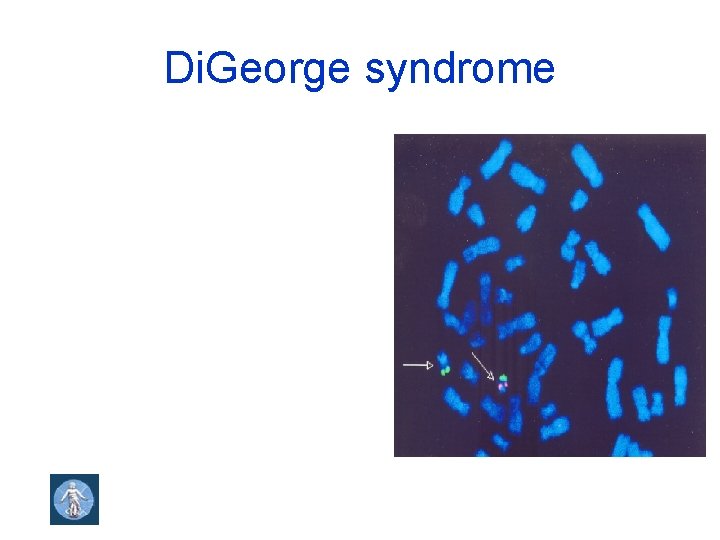
Di. George syndrome

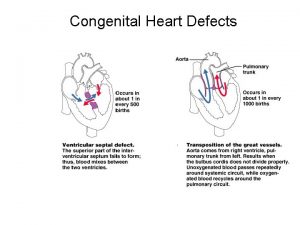
Isolated malformations / newborns • • • Anencephaly Spina bifida Hip dyslocation Atresias of esophagus and bowels Pyelectasy (obstructive uropathy) Diaphragma hernia Omphalokele Hirschsprung disease (megacolon congenitum) Congenital heart disease

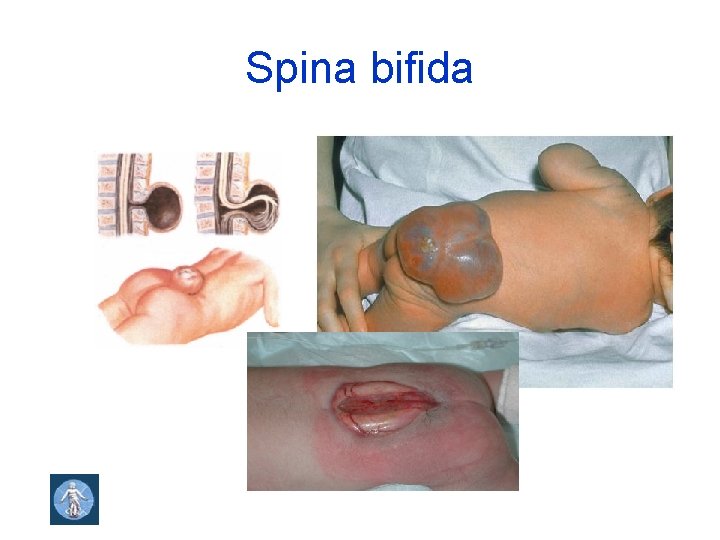
Spina bifida

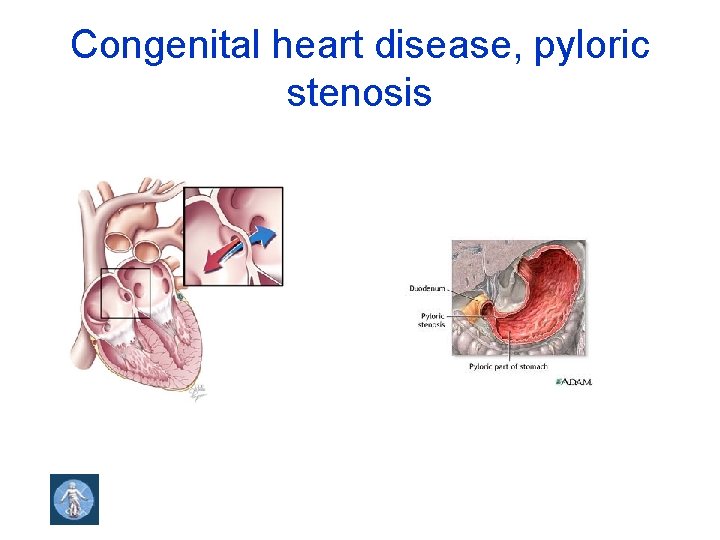
Congenital heart disease, pyloric stenosis

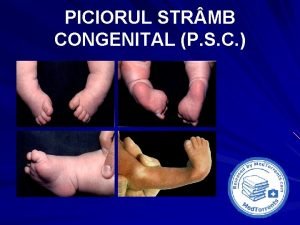
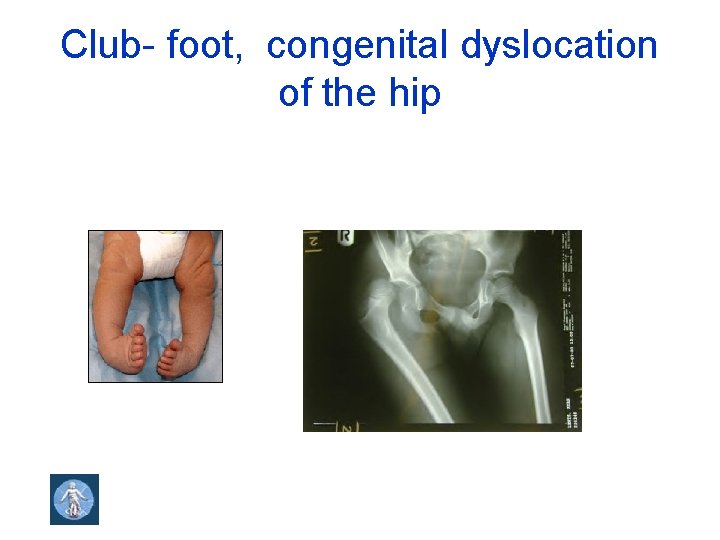
Club- foot, congenital dyslocation of the hip

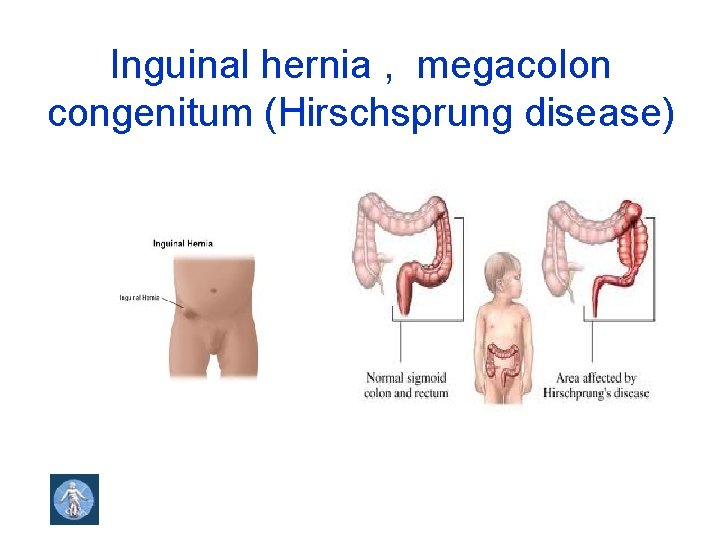
Inguinal hernia , megacolon congenitum (Hirschsprung disease)

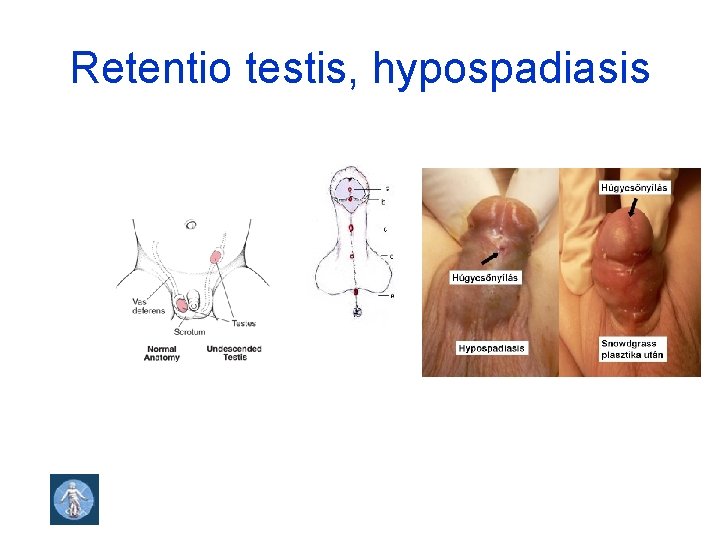
Retentio testis, hypospadiasis

Clinical signs and data of a possible chromosome aberration • Malformations, dysmorphisms of the skull and face (craniofacial dysmorphy) • Mental retardation • Multiorgan involvement • Maternal age: 35 yrs or more

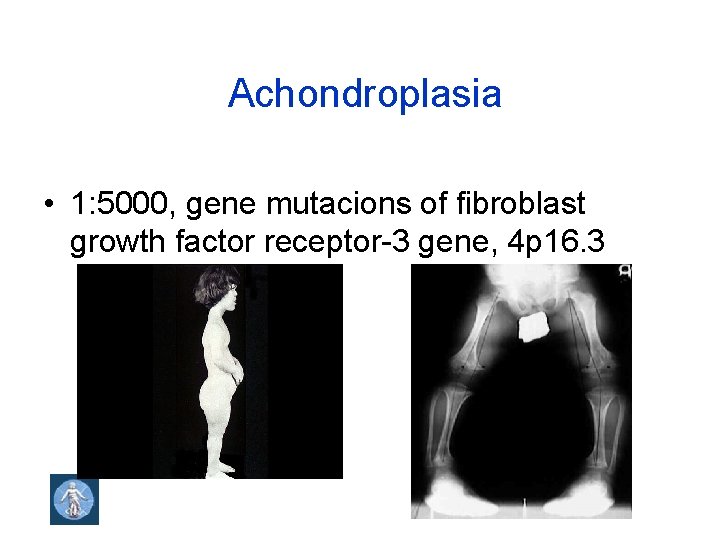
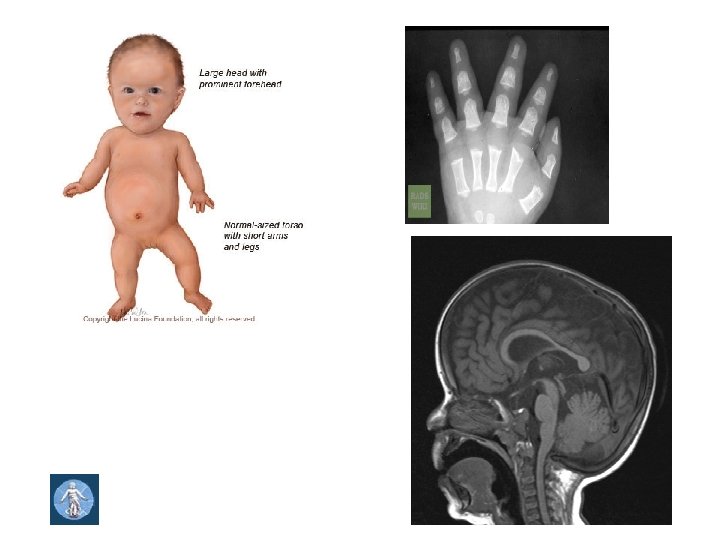
Achondroplasia • 1: 5000, gene mutacions of fibroblast growth factor receptor-3 gene, 4 p 16. 3


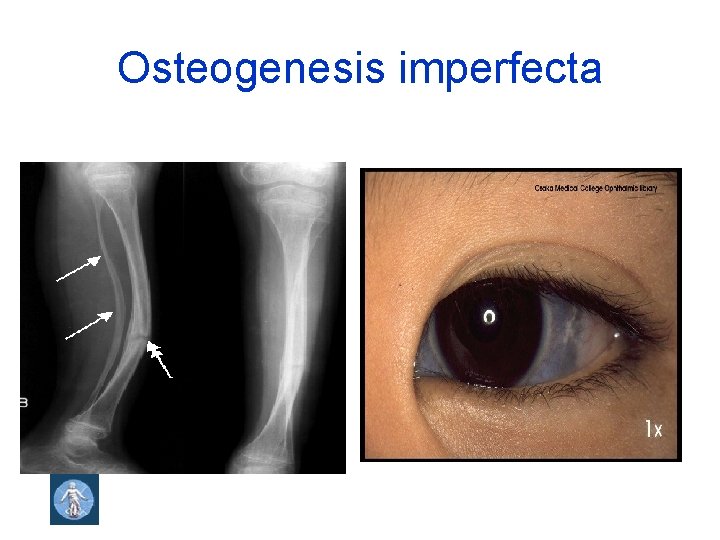
Osteogenesis imperfecta

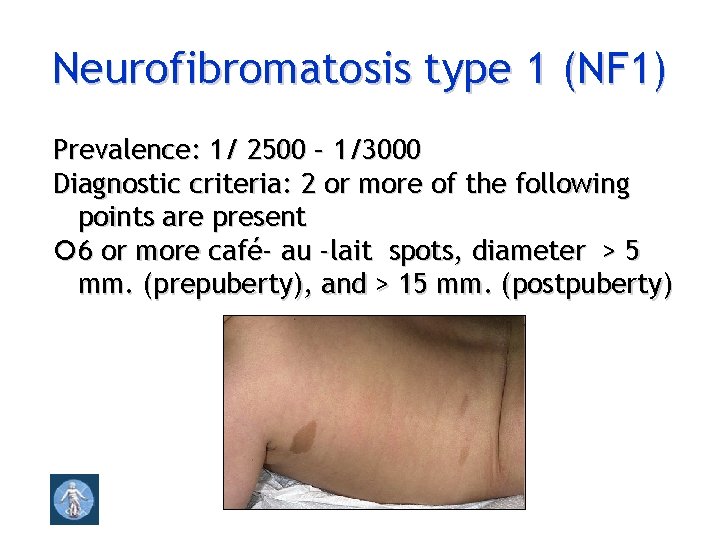
Neurofibromatosis type 1 (NF 1) Prevalence: 1/ 2500 – 1/3000 Diagnostic criteria: 2 or more of the following points are present 6 or more café- au -lait spots, diameter > 5 mm. (prepuberty), and > 15 mm. (postpuberty)

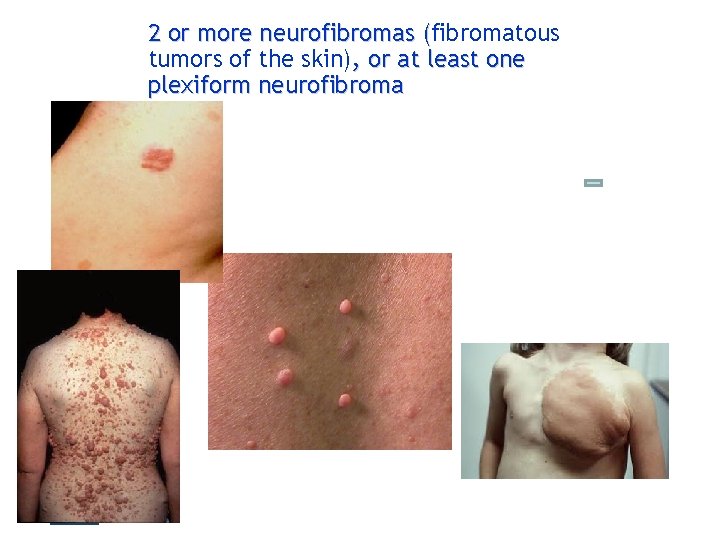
2 or more neurofibromas (fibromatous ( tumors of the skin), or at least one plexiform neurofibroma

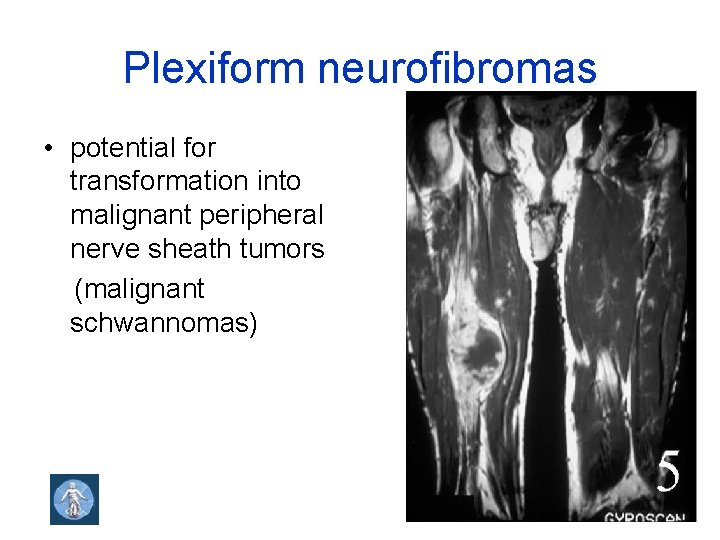
Plexiform neurofibromas • potential for transformation into malignant peripheral nerve sheath tumors (malignant schwannomas)

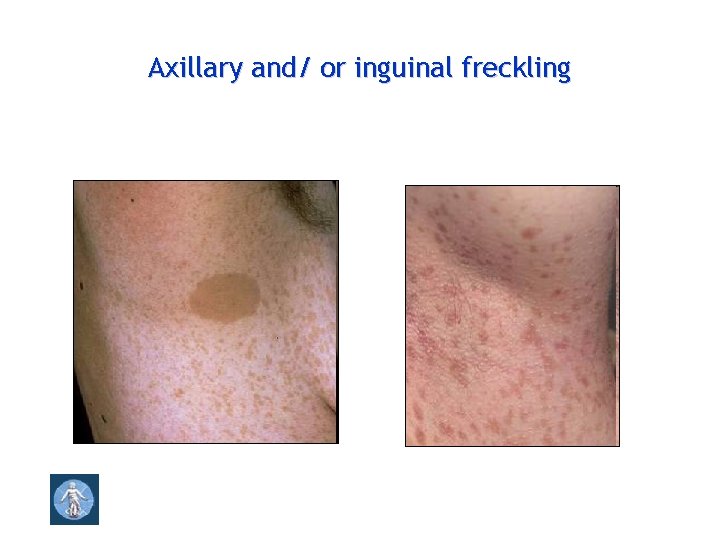
Axillary and/ or inguinal freckling

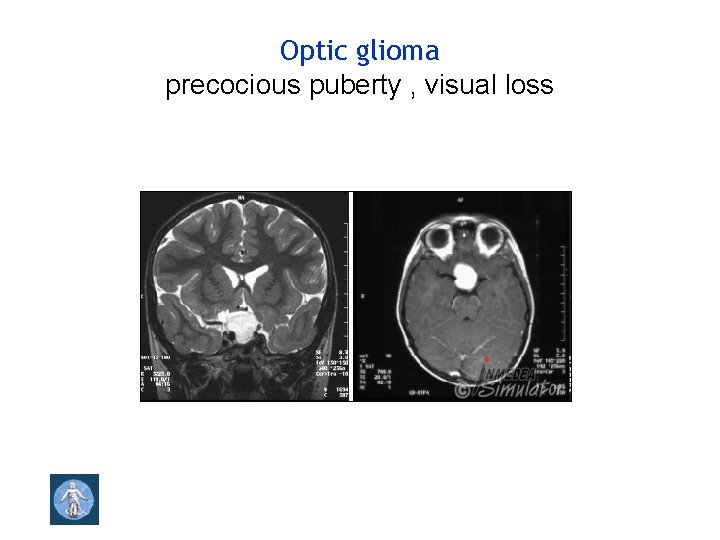
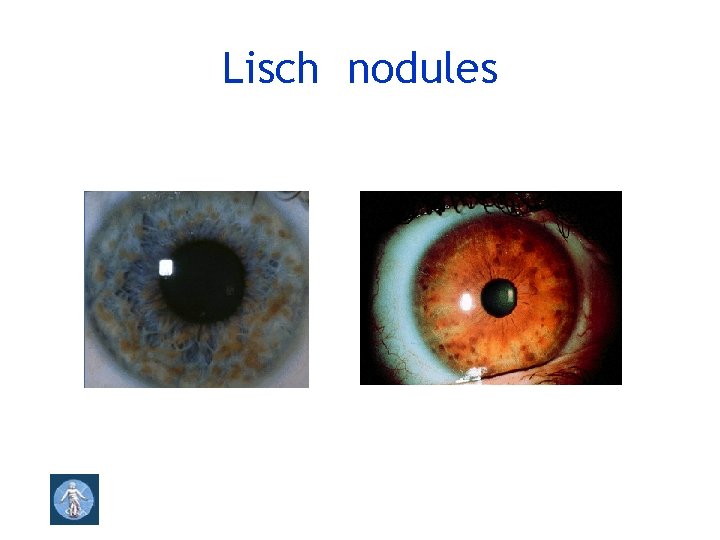
Optic pathway glioma, spinal neurofibromas 2 or more melanocytic iris hamartomas (Lisch nodules )

Optic glioma precocious puberty , visual loss

Lisch nodules

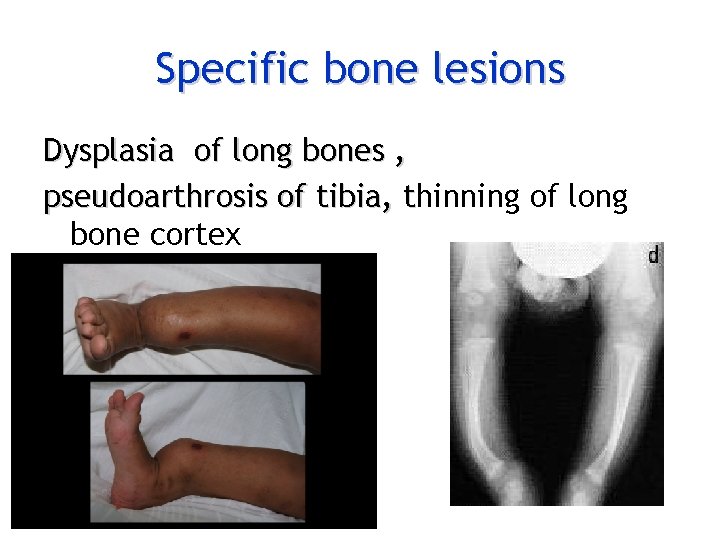
Specific bone lesions Dysplasia of long bones , pseudoarthrosis of tibia, thinning of long t bone cortex

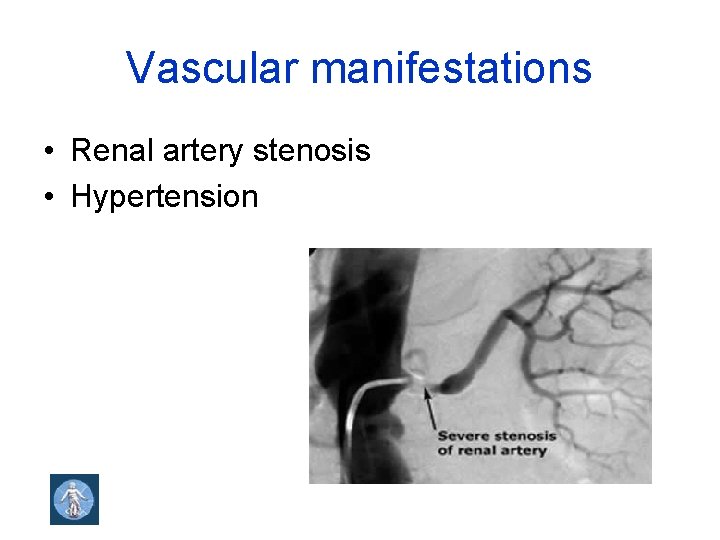
Vascular manifestations • Renal artery stenosis • Hypertension

First –degree family relative has a proven diagnosis of neurofibromatosis National Institutes of Health Consensus Conference 1987

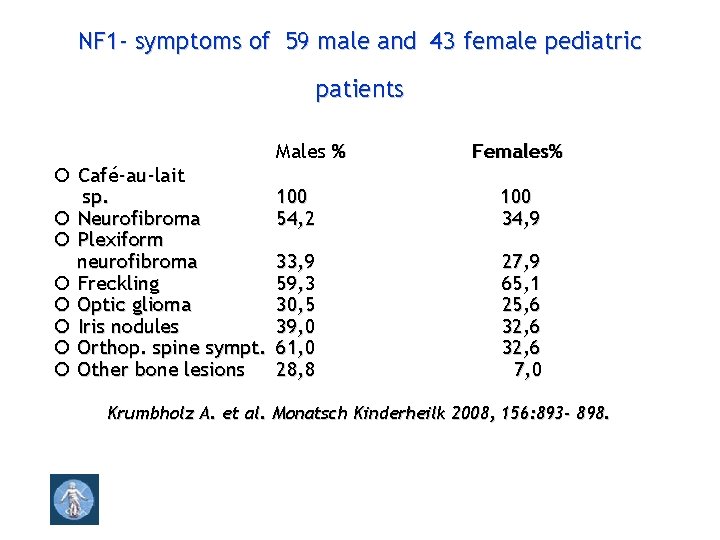
NF 1 - symptoms of 59 male and 43 female pediatric patients Café-au-lait sp. Neurofibroma Plexiform neurofibroma Freckling Optic glioma Iris nodules Orthop. spine sympt. Other bone lesions Males % Females% 100 54, 2 100 34, 9 33, 9 59, 3 30, 5 39, 0 61, 0 28, 8 27, 9 65, 1 25, 6 32, 6 7, 0 Krumbholz A. et al. Monatsch Kinderheilk 2008, 156: 893 - 898.

Some infants and children present only with „café –au – lait” spots without any other neurofibromatosis symptom and sign (innocent spots): regular pediatric observation is needed macrocephaly (occipitofrontal circumference: >97. percentile) short stature (< 3. percentile), early dentition (< 5 mo. ) : suspected NF 1 new mutation complications: learning disabilities, mental retardation, tumors (CNS, neurofibrosarcoma)

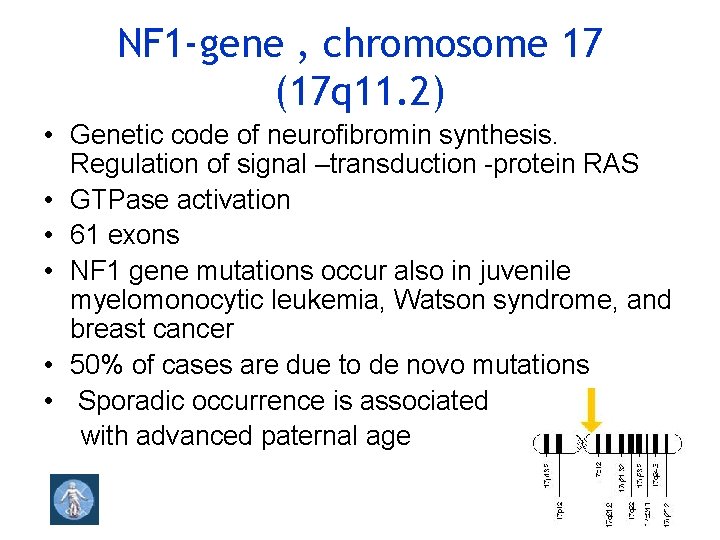
NF 1 -gene , chromosome 17 (17 q 11. 2) • Genetic code of neurofibromin synthesis. Regulation of signal –transduction -protein RAS • GTPase activation • 61 exons • NF 1 gene mutations occur also in juvenile myelomonocytic leukemia, Watson syndrome, and breast cancer • 50% of cases are due to de novo mutations • Sporadic occurrence is associated with advanced paternal age

Differential diagnosis Healthy children: 19 / 1000 newborn babies (Michalk D, Schönau E, 1999) Ovarial cysts Juvenile xanthogranuloma

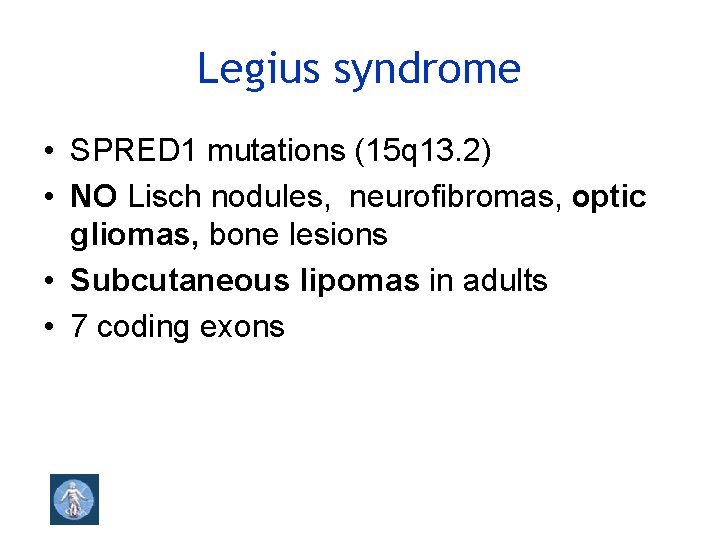
Legius syndrome • SPRED 1 mutations (15 q 13. 2) • NO Lisch nodules, neurofibromas, optic gliomas, bone lesions • Subcutaneous lipomas in adults • 7 coding exons

Cornelia de Lange (Brachmann) syndrome, symptoms • • • Microcephalia, brachycephalia Deep anterior and posterior hair border Synophrys (thick, meeting eyebrows) Ptosis, nystagmus, myopia Micrognathia long philtrum Thin lips, „carp” mouth Cheilo –gnatho - palatoschisis Malformations of limbs Hypoplastic penis, cryptorchism Mental retardation

Williams - Beuren syndromedeletion of elastin gene

K. M. female child Birth date: 02. 28. 2009. Presentation: 08. 23. 2010. Symptoms : • Somatic and psychomotoric developmental delay • Hypotonic muscles • Craniofacial dysmorphy

History • First child, healthy parents • Mother was 24, father 27 when she was born • Birth weight: 2640 g. , length: 45 cm, 39. gestational week, normal delivery , Apgar: 10/10

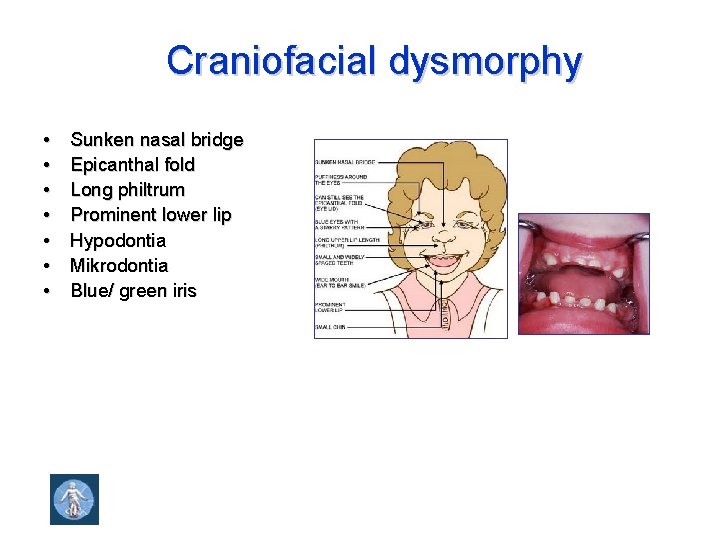
Craniofacial dysmorphy • • Sunken nasal bridge Epicanthal fold Long philtrum Prominent lower lip Hypodontia Mikrodontia Blue/ green iris

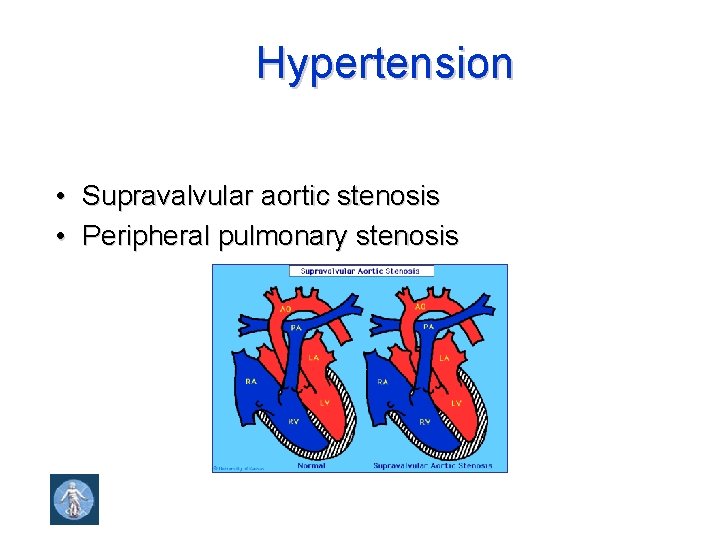
Hypertension • Supravalvular aortic stenosis • Peripheral pulmonary stenosis

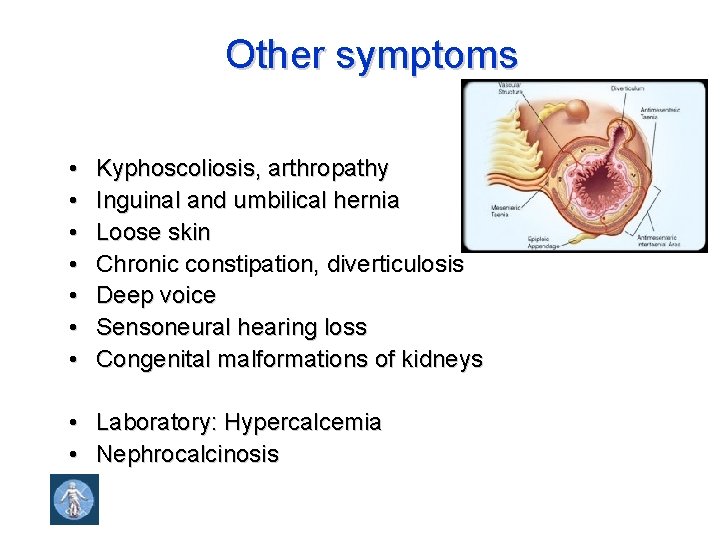
Other symptoms • • Kyphoscoliosis, arthropathy Inguinal and umbilical hernia Loose skin Chronic constipation, diverticulosis Deep voice Sensoneural hearing loss Congenital malformations of kidneys • Laboratory: Hypercalcemia • Nephrocalcinosis

Endocrinological problems • • Hypothyreoidism Early puberty Early menarche Diabetes mellitus

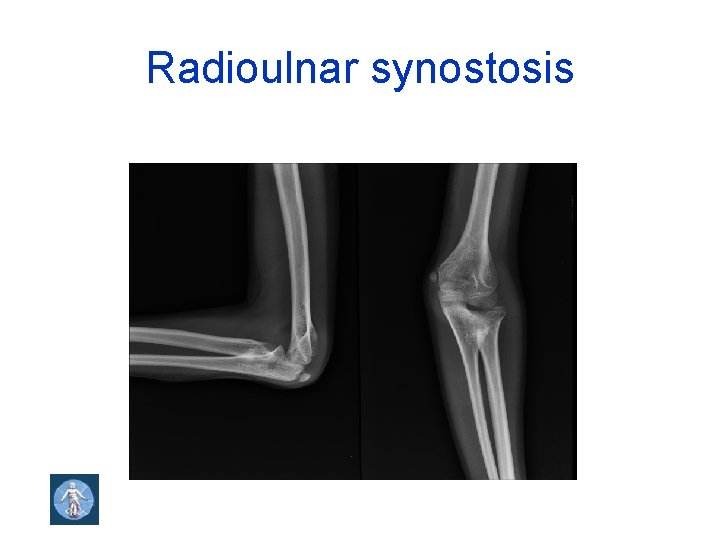
Radioulnar synostosis

• Friendly, extroverted personality, („cocktail party personality”) • Mild cognitive disturbances • Good vocabulary • Good skill to music, singing

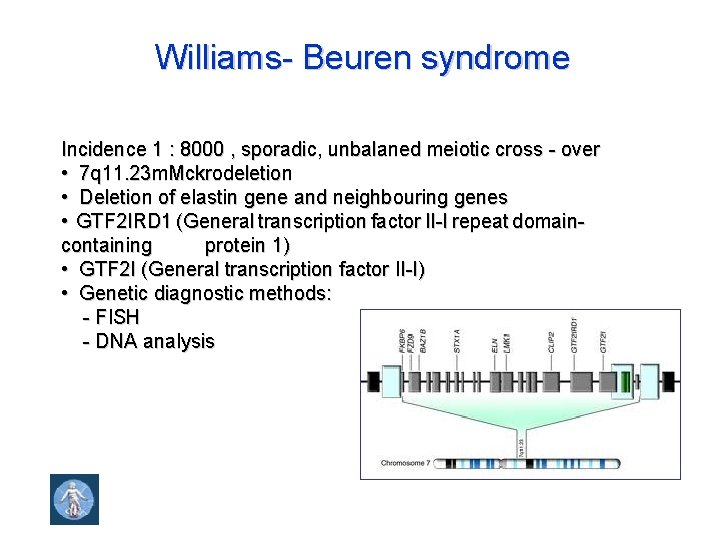
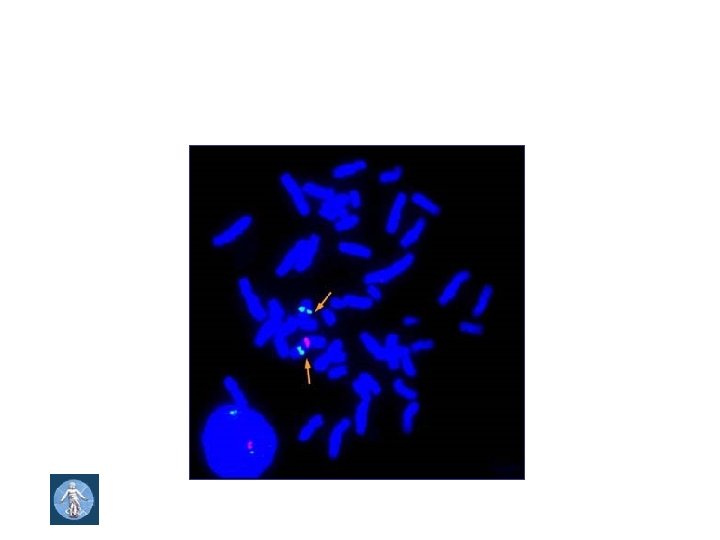
Williams- Beuren syndrome Incidence 1 : 8000 , sporadic, unbalaned meiotic cross - over • 7 q 11. 23 m. Mckrodeletion • Deletion of elastin gene and neighbouring genes • GTF 2 IRD 1 (General transcription factor II-I repeat domain- containing protein 1) • GTF 2 I (General transcription factor II-I) • Genetic diagnostic methods: - FISH - DNA analysis


• J. C. P. Williams, cardiologist, Auckland, New - Zealand, 1930 - ? Publication in: 1961 • Alois J. Beuren, cardiologist, Göttingen, 1919 -1984

Genetic counselling • • • Discussion of present and later symptoms Prognosis Pedigree analysis • Special care and support of skills • Patient organisations

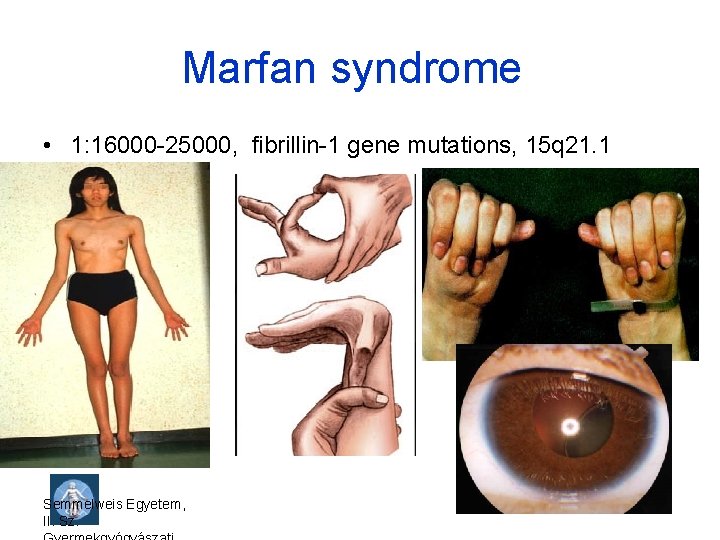
Marfan syndrome • 1: 16000 -25000, fibrillin-1 gene mutations, 15 q 21. 1 Semmelweis Egyetem, II. Sz.

Syndrome • Defects of multiple tissues • Patients’ phenotypes are similar to each other • Characteristic presentation symptoms • Clinical diagnosis is feasible in most cases

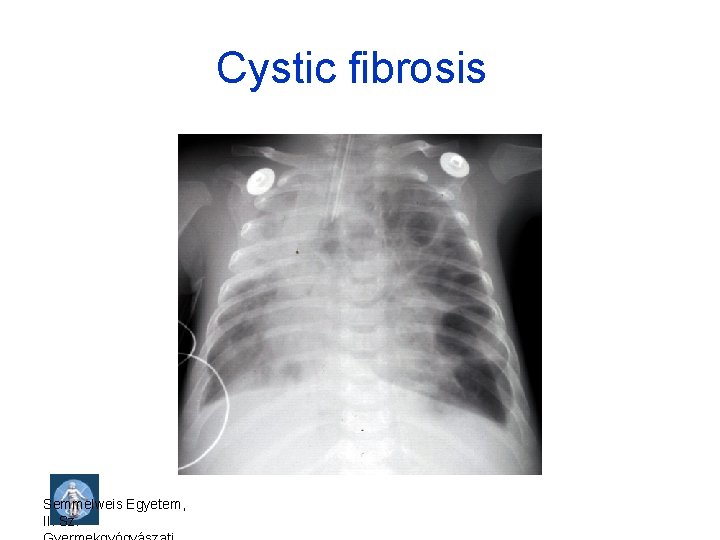
Inborn errors of metabolism • Cystic fibrosis (CF, mucoviscidosis), 1: 2500, CF transmembrane conductance regulator (CFTR) gene mutations, 7 q 31. 2 (more than 2000 mutations) • Congenital adrenal hyperplasia (CAH, adrenogenital syndrome), 1: 5000 -8000, CYP 21 gene mutations , 6 p 21. 3 Semmelweis Egyetem, II. Sz.

• Phenylketonuria (PKU, classic type, phenylalanine hydroxylase (PAH) deficiency), 1: 10000, PAH gene mutations, 12 q 22 • Galactosemia, 1: 35000 -60000, GALT gene mutations, 9 p 13 • Biotinidase deficiency, 1: 24000, BTD gene mutations, 3 p 25 • Glycogen storage diseases (von Gierke) • Mucopolysaccharidoses (Hurler/Scheie) Semmelweis Egyetem, II. Sz.

Semmelweis Egyetem, II. Sz.

Cystic fibrosis Semmelweis Egyetem, II. Sz.

Cystic fibrosis Semmelweis Egyetem, II. Sz.

Robert Guthrie (1916 - 1995) Semmelweis Egyetem, II. Sz.

Tandem mass spectrometry Separation of molecules according to their charge/ mass relation following conversion of metabolites into ions Semmelweis Egyetem, II. Sz.

Semmelweis Egyetem, II. Sz.

Hurler disease (MPS I) Semmelweis Egyetem, II. Sz.

Pompe disease

Pompe disease

Clinical signs and data of monolocus hereditary diseases • • Multiorgan involvement (syndrome) Recurrent familial occurrence Consanguinity Characteristic phenotype

Reductional malformations of limbs • Amely

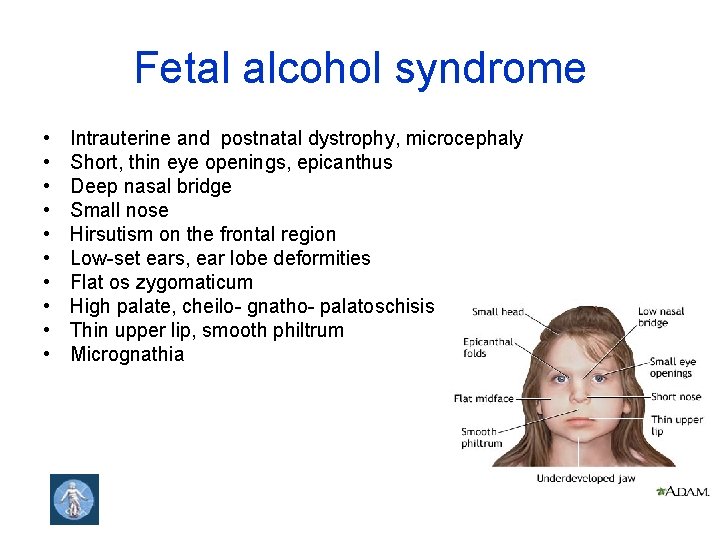
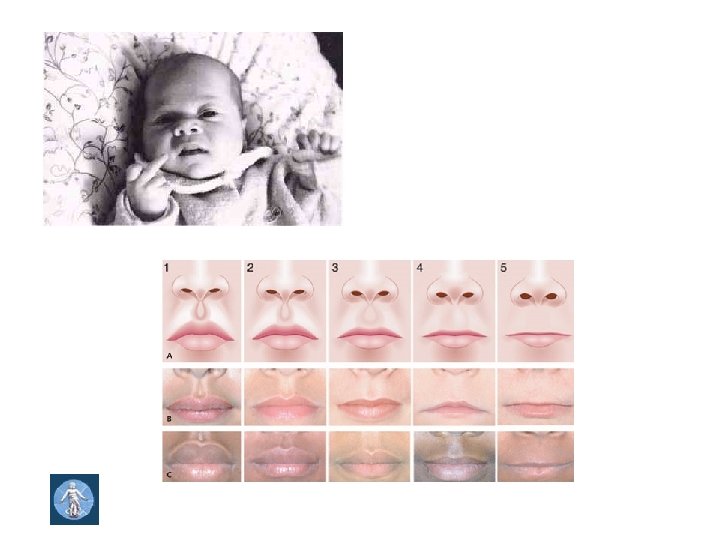
Fetal alcohol syndrome • • • Intrauterine and postnatal dystrophy, microcephaly Short, thin eye openings, epicanthus Deep nasal bridge Small nose Hirsutism on the frontal region Low-set ears, ear lobe deformities Flat os zygomaticum High palate, cheilo- gnatho- palatoschisis Thin upper lip, smooth philtrum Micrognathia


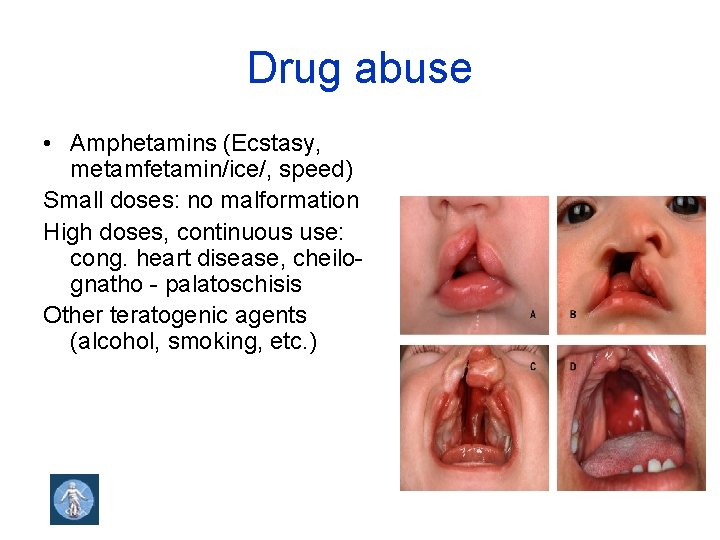
Drug abuse • Amphetamins (Ecstasy, metamfetamin/ice/, speed) Small doses: no malformation High doses, continuous use: cong. heart disease, cheilo- gnatho - palatoschisis Other teratogenic agents (alcohol, smoking, etc. )
 Congenital malformations
Congenital malformations Streptokokken
Streptokokken Phokomelien
Phokomelien Fekete csilla
Fekete csilla Ii. anund svéd király
Ii. anund svéd király Fekete árpád
Fekete árpád Jamaica a jamaicaiaké
Jamaica a jamaicaiaké Rendezvényszervezés forgatókönyv
Rendezvényszervezés forgatókönyv Fekete zab lovaknak
Fekete zab lovaknak Usgtrade
Usgtrade Dr fekete erika
Dr fekete erika Sötétben minden tehén fekete
Sötétben minden tehén fekete Dilatative kardiomyopathie
Dilatative kardiomyopathie Alexandra fekete
Alexandra fekete Dr fekete rita
Dr fekete rita Is rhotacism hereditary
Is rhotacism hereditary Hereditary hemochromatosis inheritance pattern
Hereditary hemochromatosis inheritance pattern Is intelligence hereditary
Is intelligence hereditary Jamin morrison
Jamin morrison Hereditary persistence of fetal hemoglobin
Hereditary persistence of fetal hemoglobin Why calamba called cradle of genius
Why calamba called cradle of genius Hereditary stomatocytosis
Hereditary stomatocytosis Is schizophrenia hereditary
Is schizophrenia hereditary Change in hereditary features over time
Change in hereditary features over time Hereditary units
Hereditary units C peptide low
C peptide low Hereditary spherocytosis usmle
Hereditary spherocytosis usmle Pansitopenia adalah
Pansitopenia adalah Change in hereditary features over time
Change in hereditary features over time Which type of macromolecule stores genetic information?
Which type of macromolecule stores genetic information? Hereditary stomatocytosis
Hereditary stomatocytosis Hereditary hemochromatosis
Hereditary hemochromatosis Is schizophrenia hereditary
Is schizophrenia hereditary Stores and transmits genetic (hereditary) information
Stores and transmits genetic (hereditary) information Pku is an inherited disease caused by a recessive allele
Pku is an inherited disease caused by a recessive allele Congenital rubella syndrome triad
Congenital rubella syndrome triad Congenital sinus example
Congenital sinus example Differential cyanosis
Differential cyanosis Causes of congenital anomalies
Causes of congenital anomalies Congenital neuroproliferative vestibulodynia
Congenital neuroproliferative vestibulodynia Potter face oligohydramnios
Potter face oligohydramnios Tetralogy of fallot
Tetralogy of fallot Polyrhinia
Polyrhinia Congenital pneumonia
Congenital pneumonia Ortolani galeazzi barlow
Ortolani galeazzi barlow Puberty disorder
Puberty disorder Congenital heart
Congenital heart Michael addidle
Michael addidle Choledocolithaisis
Choledocolithaisis Endocardial cushion defect
Endocardial cushion defect Cercumscribe
Cercumscribe Congenital rubella syndrome
Congenital rubella syndrome Surgical neck
Surgical neck Hymen
Hymen Vesicoureteral reflux
Vesicoureteral reflux Knee chest position
Knee chest position Hypobranchial eminence
Hypobranchial eminence Congenital amusia
Congenital amusia Picior equin definitie
Picior equin definitie Congenital rubella
Congenital rubella Icd 10 multiple congenital anomalies
Icd 10 multiple congenital anomalies Canadian congenital heart alliance
Canadian congenital heart alliance Congenital rubella syndrome triad
Congenital rubella syndrome triad Congenital
Congenital Congenital voice disorders
Congenital voice disorders Mineralocorticoid function
Mineralocorticoid function Strart
Strart Farah garmany
Farah garmany Congenital limb deficiency
Congenital limb deficiency Non classical adrenal hyperplasia
Non classical adrenal hyperplasia Hutchinson's triad
Hutchinson's triad Glaucoma management
Glaucoma management Micrognathia definition
Micrognathia definition Congenital toxoplasmosis
Congenital toxoplasmosis Glaucoma
Glaucoma Congenital adrenal hyperplasia characteristics
Congenital adrenal hyperplasia characteristics Hypothyroidism classification
Hypothyroidism classification Congenital fibrosis of the extraocular muscles
Congenital fibrosis of the extraocular muscles Congenital glaucoma
Congenital glaucoma кагами-огата
кагами-огата Graves disease mnemonic
Graves disease mnemonic Congenital heart defect
Congenital heart defect Congenital diaphragmatic hernia
Congenital diaphragmatic hernia Site:slidetodoc.com
Site:slidetodoc.com Natural history of diseases
Natural history of diseases Inflammation of mfa ppt
Inflammation of mfa ppt Diseases of the respiratory system
Diseases of the respiratory system What are periradicular tissues?
What are periradicular tissues? Seborrheic keratoses
Seborrheic keratoses Std
Std