HER 2 CLIMB Tucatinib Plus Trastuzumab and Capecitabine

HER 2 CLIMB: Tucatinib Plus Trastuzumab and Capecitabine in Previously Treated HER 2 -Positive MBC With Brain Metastases CCO Independent Conference Coverage* Highlights of the 2020 ASCO Virtual Scientific Meeting, May 29 -31, 2020 *CCO is an independent medical education company that provides state-of-the-art medical information to healthcare professionals through conference coverage and other educational programs. This activity is provided by Clinical Care Options, LLC Supported by educational grants from Glaxo. Smith. Kline LLC, Merck Sharp & Dohme Corp. , and Novartis Pharmaceuticals Corporation
About These Slides § Please feel free to use, update, and share some or all of these slides in your noncommercial presentations to colleagues or patients § When using our slides, please retain the source attribution: Slide credit: clinicaloptions. com § These slides may not be published, posted online, or used in commercial presentations without permission. Please contact permissions@clinicaloptions. com for details
HER 2 CLIMB Intracranial Activity: Background § Brain metastases develop in close to half of patients with HER 2+ MBC[1 -3] § In primary analysis of phase II HER 2 CLIMB trial, addition of tucatinib to trastuzumab + capecitabine significantly improved OS and PFS for patients with HER 2+ MBC, including those with brain metastases[4] ‒ Tucatinib: oral TKI selective for HER 2 with minimal inhibition of EGFR [5, 6] ‒ HR for PFS: 0. 54 (95% CI: 0. 42 -0. 71; P <. 001); HR for OS: 0. 66 (95% CI: 0. 50 -0. 88; P =. 005) ‒ HR for PFS in patients with brain mets: 0. 48 (95% CI: 0. 34 -0. 69; P <. 001) ‒ Tucatinib recently approved in combination with trastuzumab and capecitabine for unresectable or metastatic HER 2+ BC following at least 1 prior HER 2 -targeted therapy [7] § Current exploratory analysis evaluated intracranial efficacy, survival of adding tucatinib vs placebo to trastuzumab/capecitabine in patients with baseline brain metastases [8, 9] 1. Bendell. Cancer. 2003; 97: 2972. 2. Leyland-Jones. JCO. 2009; 27: 5278. 3. Olson. Breast. 2013; 22: 525. 4. Murthy. NEJM. 2020; 382: 597. 5. Pheneger. Slide credit: clinicaloptions. com Cancer Res. 2009; 69: 1795. 6. Kulukian. SABCS 2019. Abstr P 1 -18 -09. 7. Tucatinib PI. 8. Lin. ASCO 2020. Abstr 1005. 9. Lin. JCO. 2020; [Epub].
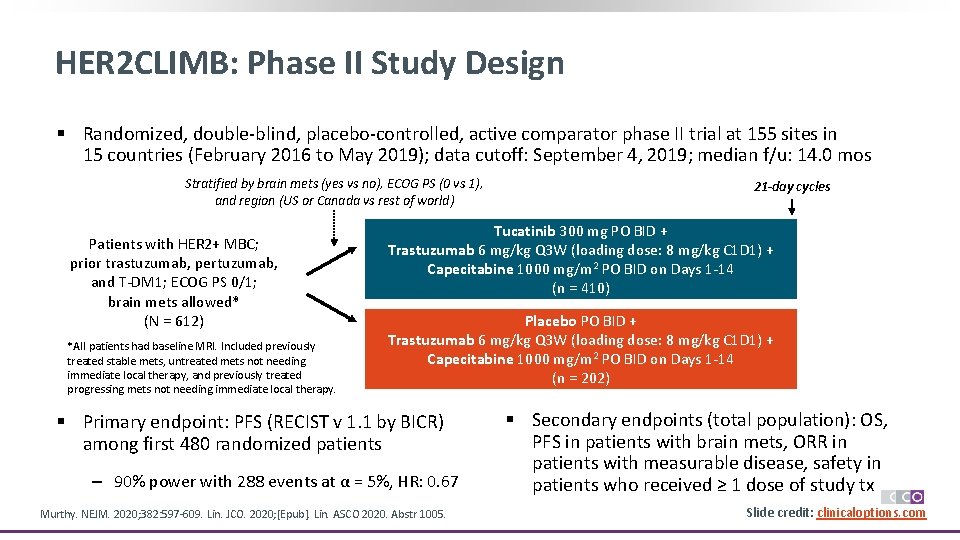
HER 2 CLIMB: Phase II Study Design § Randomized, double-blind, placebo-controlled, active comparator phase II trial at 155 sites in 15 countries (February 2016 to May 2019); data cutoff: September 4, 2019; median f/u: 14. 0 mos Stratified by brain mets (yes vs no), ECOG PS (0 vs 1), and region (US or Canada vs rest of world) Patients with HER 2+ MBC; prior trastuzumab, pertuzumab, and T-DM 1; ECOG PS 0/1; brain mets allowed* (N = 612) *All patients had baseline MRI. Included previously treated stable mets, untreated mets not needing immediate local therapy, and previously treated progressing mets not needing immediate local therapy. 21 -day cycles Tucatinib 300 mg PO BID + Trastuzumab 6 mg/kg Q 3 W (loading dose: 8 mg/kg C 1 D 1) + Capecitabine 1000 mg/m 2 PO BID on Days 1 -14 (n = 410) Placebo PO BID + Trastuzumab 6 mg/kg Q 3 W (loading dose: 8 mg/kg C 1 D 1) + Capecitabine 1000 mg/m 2 PO BID on Days 1 -14 (n = 202) § Primary endpoint: PFS (RECIST v 1. 1 by BICR) among first 480 randomized patients ‒ 90% power with 288 events at α = 5%, HR: 0. 67 Murthy. NEJM. 2020; 382: 597 -609. Lin. JCO. 2020; [Epub]. Lin. ASCO 2020. Abstr 1005. § Secondary endpoints (total population): OS, PFS in patients with brain mets, ORR in patients with measurable disease, safety in patients who received ≥ 1 dose of study tx Slide credit: clinicaloptions. com
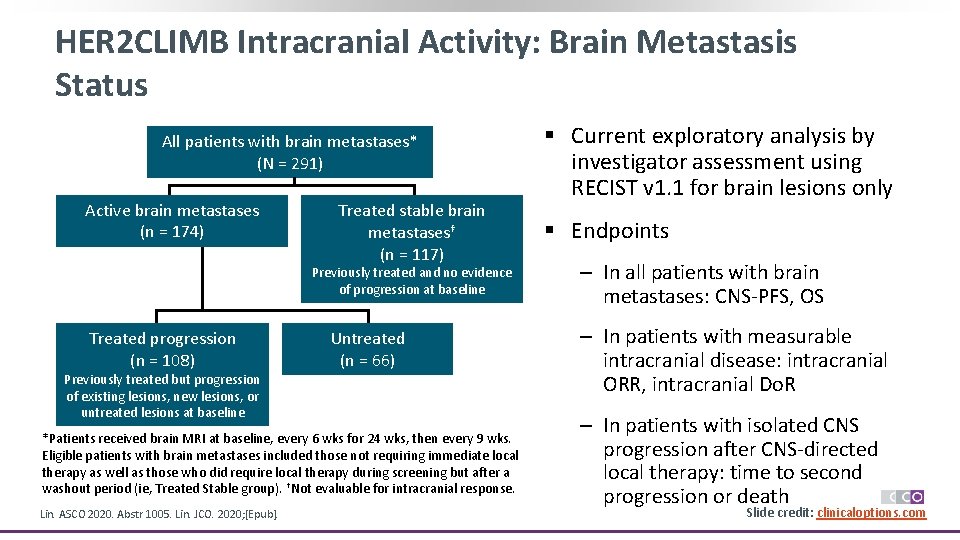
HER 2 CLIMB Intracranial Activity: Brain Metastasis Status All patients with brain metastases* (N = 291) Active brain metastases (n = 174) Treated stable brain metastases† (n = 117) Previously treated and no evidence of progression at baseline Treated progression (n = 108) Previously treated but progression of existing lesions, new lesions, or untreated lesions at baseline Untreated (n = 66) *Patients received brain MRI at baseline, every 6 wks for 24 wks, then every 9 wks. Eligible patients with brain metastases included those not requiring immediate local therapy as well as those who did require local therapy during screening but after a washout period (ie, Treated Stable group). †Not evaluable for intracranial response. Lin. ASCO 2020. Abstr 1005. Lin. JCO. 2020; [Epub]. § Current exploratory analysis by investigator assessment using RECIST v 1. 1 for brain lesions only § Endpoints ‒ In all patients with brain metastases: CNS-PFS, OS ‒ In patients with measurable intracranial disease: intracranial ORR, intracranial Do. R ‒ In patients with isolated CNS progression after CNS-directed local therapy: time to second progression or death Slide credit: clinicaloptions. com
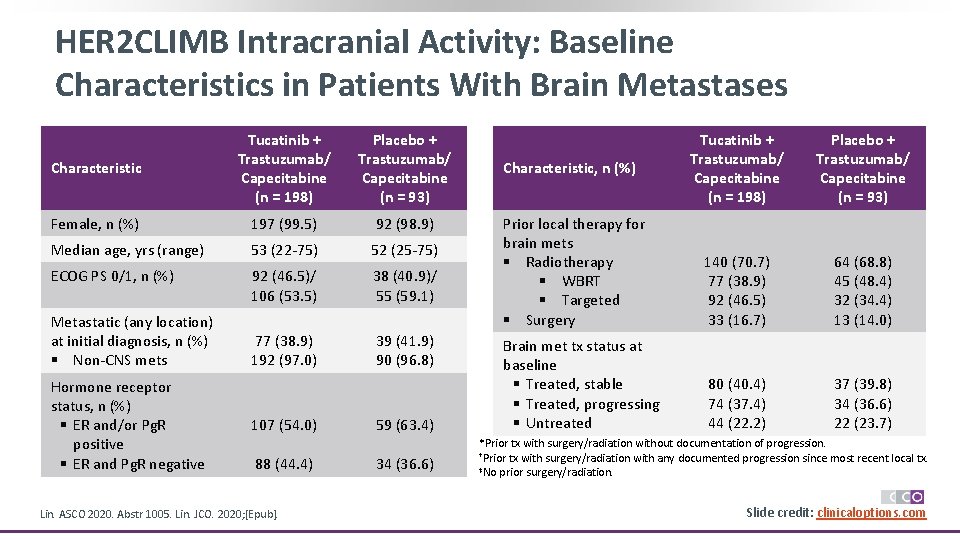
HER 2 CLIMB Intracranial Activity: Baseline Characteristics in Patients With Brain Metastases Characteristic Tucatinib + Trastuzumab/ Capecitabine (n = 198) Placebo + Trastuzumab/ Capecitabine (n = 93) Female, n (%) 197 (99. 5) 92 (98. 9) Median age, yrs (range) 53 (22 -75) 52 (25 -75) ECOG PS 0/1, n (%) 92 (46. 5)/ 106 (53. 5) 38 (40. 9)/ 55 (59. 1) 77 (38. 9) 192 (97. 0) 39 (41. 9) 90 (96. 8) Metastatic (any location) at initial diagnosis, n (%) § Non-CNS mets Hormone receptor status, n (%) § ER and/or Pg. R positive § ER and Pg. R negative 107 (54. 0) 88 (44. 4) Lin. ASCO 2020. Abstr 1005. Lin. JCO. 2020; [Epub]. 59 (63. 4) 34 (36. 6) Tucatinib + Trastuzumab/ Capecitabine (n = 198) Placebo + Trastuzumab/ Capecitabine (n = 93) Prior local therapy for brain mets § Radiotherapy § WBRT § Targeted § Surgery 140 (70. 7) 77 (38. 9) 92 (46. 5) 33 (16. 7) 64 (68. 8) 45 (48. 4) 32 (34. 4) 13 (14. 0) Brain met tx status at baseline § Treated, stable § Treated, progressing § Untreated 80 (40. 4) 74 (37. 4) 44 (22. 2) 37 (39. 8) 34 (36. 6) 22 (23. 7) Characteristic, n (%) *Prior tx with surgery/radiation without documentation of progression. †Prior tx with surgery/radiation with any documented progression since most recent local tx. ‡No prior surgery/radiation. Slide credit: clinicaloptions. com
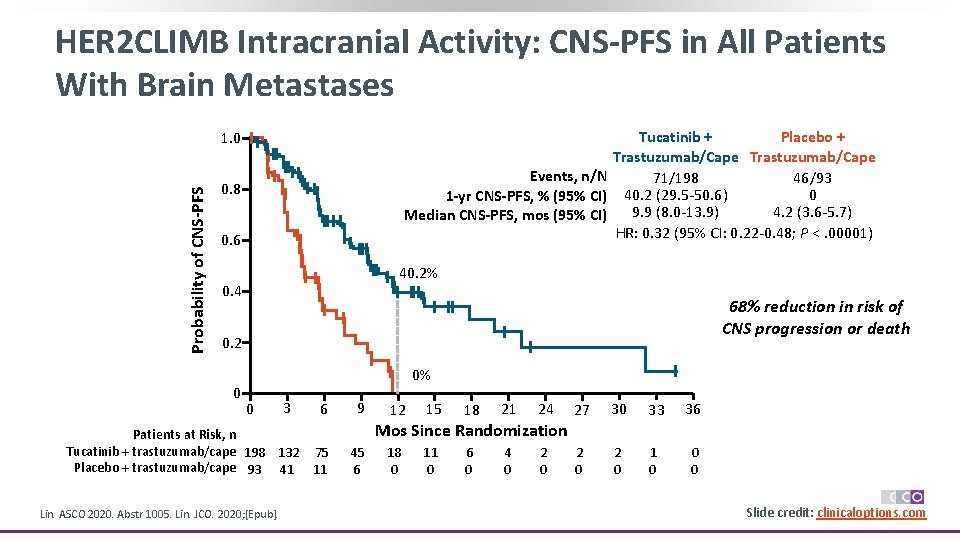
HER 2 CLIMB Intracranial Activity: CNS-PFS in All Patients With Brain Metastases Tucatinib + Placebo + Trastuzumab/Cape Events, n/N 71/198 46/93 0 1 -yr CNS-PFS, % (95% CI) 40. 2 (29. 5 -50. 6) 9. 9 (8. 0 -13. 9) 4. 2 (3. 6 -5. 7) Median CNS-PFS, mos (95% CI) HR: 0. 32 (95% CI: 0. 22 -0. 48; P <. 00001) Probability of CNS-PFS 1. 0 0. 8 0. 6 40. 2% 0. 4 68% reduction in risk of CNS progression or death 0. 2 0 0% 0 3 Patients at Risk, n Tucatinib + trastuzumab/cape 198 132 Placebo + trastuzumab/cape 93 41 Lin. ASCO 2020. Abstr 1005. Lin. JCO. 2020; [Epub]. 6 9 12 15 18 21 24 18 0 11 0 6 0 4 0 2 0 Mos Since Randomization 75 11 45 6 27 30 33 36 2 0 1 0 0 0 Slide credit: clinicaloptions. com
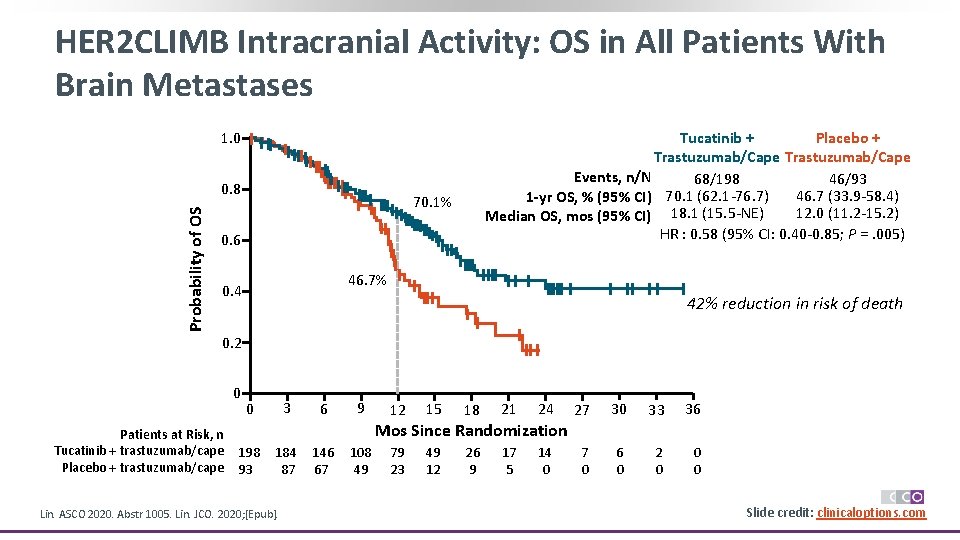
HER 2 CLIMB Intracranial Activity: OS in All Patients With Brain Metastases 1. 0 Probability of OS 0. 8 Tucatinib + Placebo + Trastuzumab/Cape Events, n/N 68/198 46/93 46. 7 (33. 9 -58. 4) 1 -yr OS, % (95% CI) 70. 1 (62. 1 -76. 7) 12. 0 (11. 2 -15. 2) Median OS, mos (95% CI) 18. 1 (15. 5 -NE) HR : 0. 58 (95% CI: 0. 40 -0. 85; P =. 005) 70. 1% 0. 6 46. 7% 0. 4 42% reduction in risk of death 0. 2 0 Patients at Risk, n Tucatinib + trastuzumab/cape Placebo + trastuzumab/cape 3 0 6 9 12 15 18 21 24 79 23 49 12 26 9 17 5 14 0 Mos Since Randomization 198 93 184 87 Lin. ASCO 2020. Abstr 1005. Lin. JCO. 2020; [Epub]. 146 67 108 49 27 30 33 36 7 0 6 0 2 0 0 0 Slide credit: clinicaloptions. com
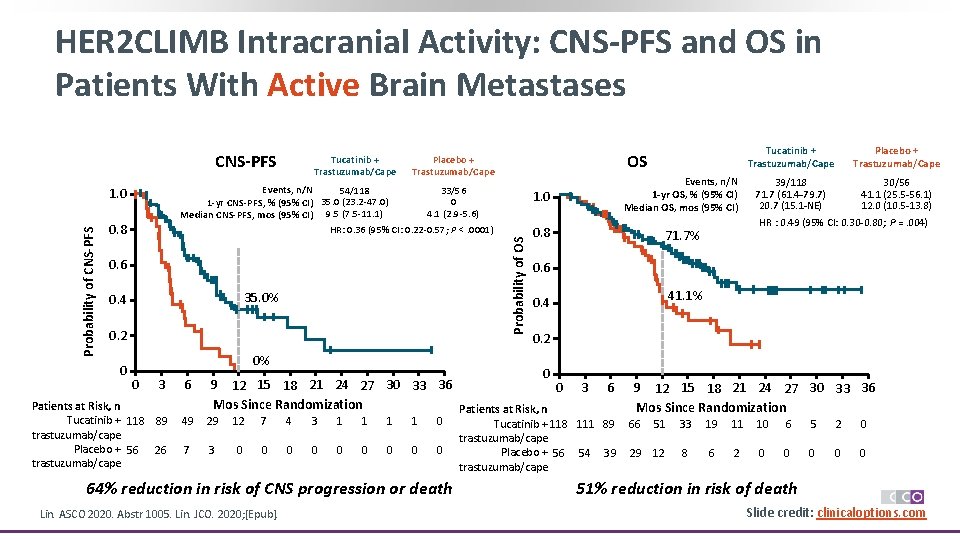
HER 2 CLIMB Intracranial Activity: CNS-PFS and OS in Patients With Active Brain Metastases CNS-PFS Probability of OS 0. 8 0. 6 35. 0% 0. 4 0. 2 0 Tucatinib + Trastuzumab/Cape OS Placebo + Trastuzumab/Cape Events, n/N 54/118 33/56 0 1 -yr CNS-PFS, % (95% CI) 35. 0 (23. 2 -47. 0) 4. 1 (2. 9 -5. 6) Median CNS-PFS, mos (95% CI) 9. 5 (7. 5 -11. 1) HR: 0. 36 (95% CI: 0. 22 -0. 57; P <. 0001) 1. 0 Probability of CNS-PFS Tucatinib + Trastuzumab/Cape 1. 0 Events, n/N 1 -yr OS, % (95% CI) Median OS, mos (95% CI) 0. 8 71. 7% Placebo + Trastuzumab/Cape 39/118 30/56 71. 7 (61. 4 -79. 7) 41. 1 (25. 5 -56. 1) 20. 7 (15. 1 -NE) 12. 0 (10. 5 -13. 8) HR : 0. 49 (95% CI: 0. 30 -0. 80; P =. 004) 0. 6 41. 1% 0. 4 0. 2 0% 0 3 Patients at Risk, n Tucatinib + 118 89 trastuzumab/cape Placebo + 56 26 trastuzumab/cape 6 0 9 12 15 18 21 24 27 30 33 36 0 Mos Since Randomization Patients at Risk, n 49 29 12 7 4 3 1 1 0 7 3 0 0 0 0 0 64% reduction in risk of CNS progression or death Lin. ASCO 2020. Abstr 1005. Lin. JCO. 2020; [Epub]. 3 6 Tucatinib + 118 111 89 trastuzumab/cape Placebo + 56 54 39 trastuzumab/cape 9 12 15 18 21 24 27 30 33 36 Mos Since Randomization 66 51 29 12 33 19 11 10 6 5 2 0 8 6 2 0 0 0 51% reduction in risk of death Slide credit: clinicaloptions. com
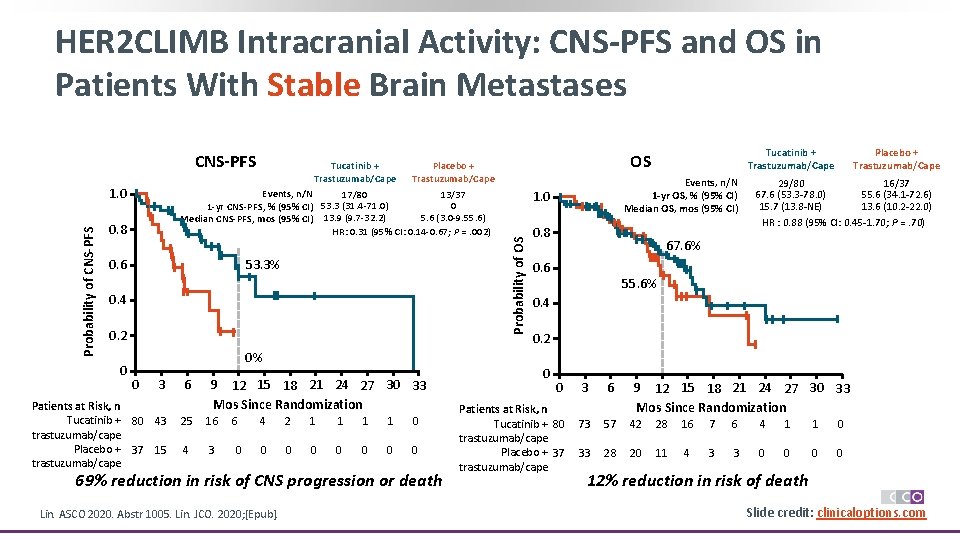
HER 2 CLIMB Intracranial Activity: CNS-PFS and OS in Patients With Stable Brain Metastases Probability of CNS-PFS 1. 0 Tucatinib + Trastuzumab/Cape Placebo + Trastuzumab/Cape Events, n/N 17/80 13/37 0 1 -yr CNS-PFS, % (95% CI) 53. 3 (31. 4 -71. 0) 5. 6 (3. 0 -9. 55. 6) Median CNS-PFS, mos (95% CI) 13. 9 (9. 7 -32. 2) HR: 0. 31 (95% CI: 0. 14 -0. 67; P =. 002) 0. 8 53. 3% 0. 6 0. 4 0. 2 0 0% 0 3 Patients at Risk, n Tucatinib + 80 43 trastuzumab/cape Placebo + 37 15 trastuzumab/cape 6 9 12 15 18 21 24 27 30 33 Mos Since Randomization 25 16 4 3 6 0 4 2 1 1 0 0 0 0 69% reduction in risk of CNS progression or death Lin. ASCO 2020. Abstr 1005. Lin. JCO. 2020; [Epub]. Tucatinib + Trastuzumab/Cape OS Events, n/N 1 -yr OS, % (95% CI) Median OS, mos (95% CI) 1. 0 Probability of OS CNS-PFS 0. 8 Placebo + Trastuzumab/Cape 29/80 16/37 67. 6 (53. 3 -78. 0) 55. 6 (34. 1 -72. 6) 15. 7 (13. 8 -NE) 13. 6 (10. 2 -22. 0) HR : 0. 88 (95% CI: 0. 45 -1. 70; P =. 70) 67. 6% 0. 6 55. 6% 0. 4 0. 2 0 0 Patients at Risk, n Tucatinib + 80 trastuzumab/cape Placebo + 37 trastuzumab/cape 3 6 9 12 15 18 21 24 27 30 33 Mos Since Randomization 73 57 42 28 16 7 6 4 1 1 0 33 28 20 11 4 3 3 0 0 12% reduction in risk of death Slide credit: clinicaloptions. com
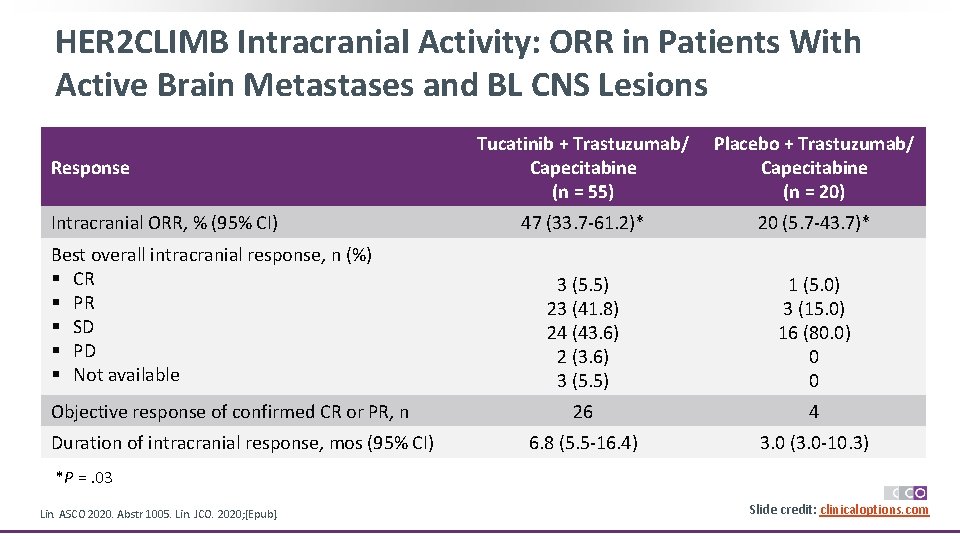
HER 2 CLIMB Intracranial Activity: ORR in Patients With Active Brain Metastases and BL CNS Lesions Response Intracranial ORR, % (95% CI) Best overall intracranial response, n (%) § CR § PR § SD § PD § Not available Objective response of confirmed CR or PR, n Duration of intracranial response, mos (95% CI) Tucatinib + Trastuzumab/ Capecitabine (n = 55) Placebo + Trastuzumab/ Capecitabine (n = 20) 47 (33. 7 -61. 2)* 20 (5. 7 -43. 7)* 3 (5. 5) 23 (41. 8) 24 (43. 6) 2 (3. 6) 3 (5. 5) 1 (5. 0) 3 (15. 0) 16 (80. 0) 0 0 26 4 6. 8 (5. 5 -16. 4) 3. 0 (3. 0 -10. 3) *P =. 03 Lin. ASCO 2020. Abstr 1005. Lin. JCO. 2020; [Epub]. Slide credit: clinicaloptions. com
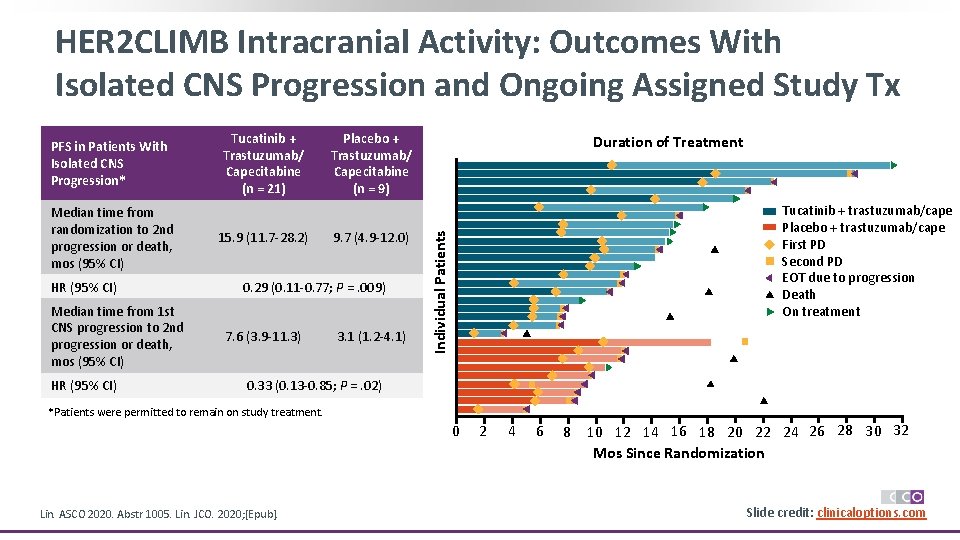
HER 2 CLIMB Intracranial Activity: Outcomes With Isolated CNS Progression and Ongoing Assigned Study Tx Median time from randomization to 2 nd progression or death, mos (95% CI) HR (95% CI) Median time from 1 st CNS progression to 2 nd progression or death, mos (95% CI) HR (95% CI) Tucatinib + Trastuzumab/ Capecitabine (n = 21) 15. 9 (11. 7 -28. 2) Placebo + Trastuzumab/ Capecitabine (n = 9) 9. 7 (4. 9 -12. 0) 0. 29 (0. 11 -0. 77; P =. 009) 7. 6 (3. 9 -11. 3) 3. 1 (1. 2 -4. 1) Duration of Treatment Tucatinib + trastuzumab/cape Placebo + trastuzumab/cape First PD Second PD EOT due to progression Death On treatment Individual Patients PFS in Patients With Isolated CNS Progression* 0. 33 (0. 13 -0. 85; P =. 02) *Patients were permitted to remain on study treatment. 0 Lin. ASCO 2020. Abstr 1005. Lin. JCO. 2020; [Epub]. 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 Mos Since Randomization Slide credit: clinicaloptions. com
HER 2 CLIMB Intracranial Activity: Conclusions § Tucatinib + trastuzumab/capecitabine demonstrated statistically significant and clinically meaningful improvements in intracranial ORR, CNS-PFS, and OS ‒ 68% reduction in risk of CNS progression, 42% reduction in risk of death ‒ CNS-PFS and OS benefit observed in patients with active brain mets § Current study is first RCT to report a TKI leading to prolonged OS in patients with HER 2+ MBC and brain mets § Taken together with HER 2 CLIMB primary analysis, investigators conclude this regimen is active against intracranial and extracranial disease in HER 2+ MBC Lin. ASCO 2020. Abstr 1005. Lin. JCO. 2020; [Epub]. Slide credit: clinicaloptions. com

Go Online for More CCO Coverage of ASCO 2020! Short slideset summaries and additional CME-certified analyses with expert faculty commentary on key studies in: § Breast cancer § Gynecologic cancers § Gastrointestinal cancers § Hematologic malignancies § Genitourinary cancers § Lung cancer clinicaloptions. com/oncology
- Slides: 14