HEPATITIS Sahar Zakaria Lecterer of Tropical Medicine Clinical






























- Slides: 56

HEPATITIS Sahar Zakaria Lecterer of Tropical Medicine

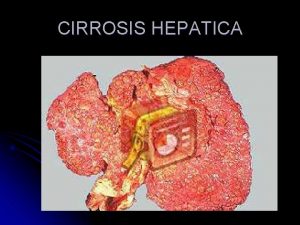
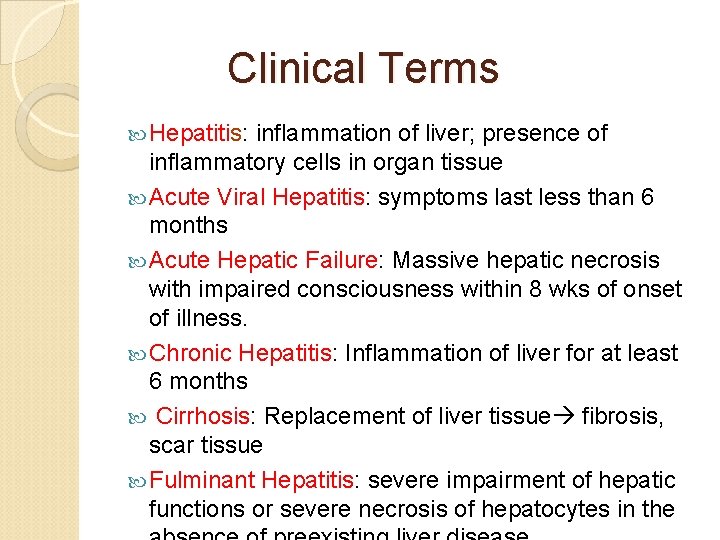
Clinical Terms Hepatitis: inflammation of liver; presence of inflammatory cells in organ tissue Acute Viral Hepatitis: symptoms last less than 6 months Acute Hepatic Failure: Massive hepatic necrosis with impaired consciousness within 8 wks of onset of illness. Chronic Hepatitis: Inflammation of liver for at least 6 months Cirrhosis: Replacement of liver tissue fibrosis, scar tissue Fulminant Hepatitis: severe impairment of hepatic functions or severe necrosis of hepatocytes in the

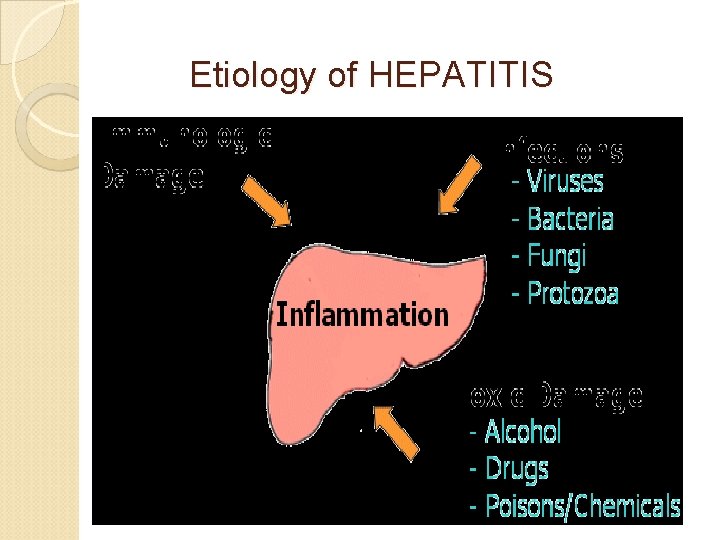
Etiology of HEPATITIS

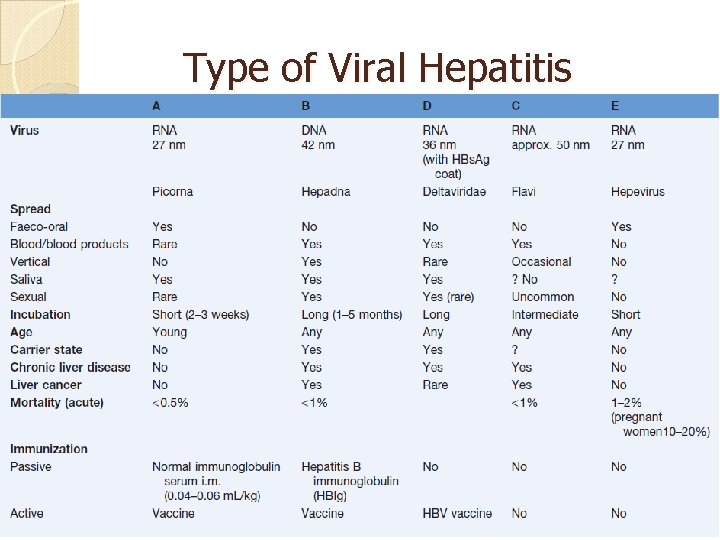
Type of Viral Hepatitis

Classic presentation: Acute Viral Hepatitis Phase 1 - Viral replication; Patients are asymptomatic during this phase. Phase 2 –. Prodromal phase: Patients experience anorexia, nausea, vomiting, alterations in taste, arthralgias, malaise, fatigue, urticaria, and pruritus. Some develop an aversion to cigarette smoke. When seen by a health care provider during this phase, patients are often diagnosed as having gastroenteritis or a viral syndrome.

Phase 3 - Icteric phase : Jaundice, Patients may note dark urine, followed by pale-colored stools. In addition to the predominant gastrointestinal symptoms and malaise, patients become icteric and may develop right upper quadrant pain with hepatomegaly. Severe cases may result in Fulminant Hepatitis: 1. Hepatic Encephalopathy: B/L asterixis, palmar erythema 2. Hepatorenal syndrome 3. Bleeding diathesis Phase 4 - Convalescent phase; symptoms and icterus resolve. Liver enzymes return to normal.

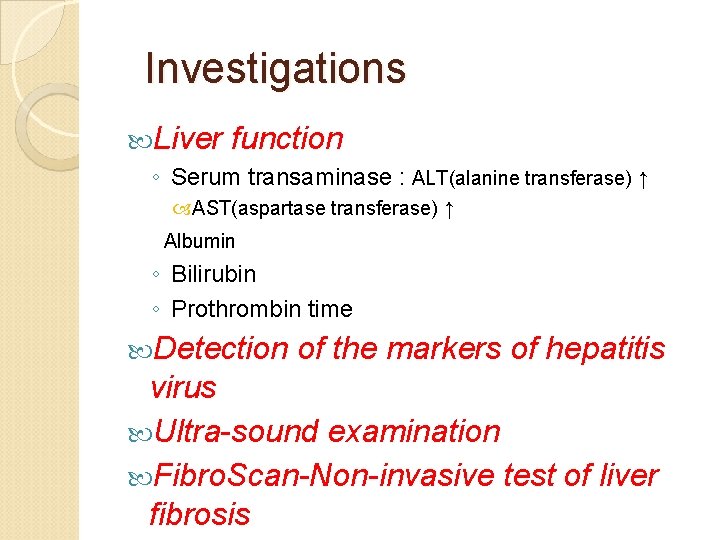
Investigations Liver function ◦ Serum transaminase : ALT(alanine transferase) ↑ AST(aspartase transferase) ↑ Albumin ◦ Bilirubin ◦ Prothrombin time Detection of the markers of hepatitis virus Ultra-sound examination Fibro. Scan-Non-invasive test of liver fibrosis

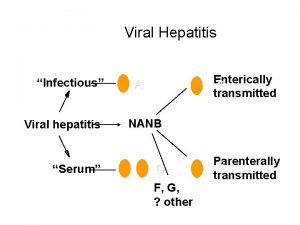
Hepatitis A virus

Hepatitis A Common cause of acute hepatitis Single-stranded, positive-sense, linear RNA enterovirus (Picornaviridae) Transmission: faecal-oral Incubation: 2 -6 weeks High-risk countries: Eastern Europe, Africa, Asia, South America The proportion of symptomatic forms and complications increase with age

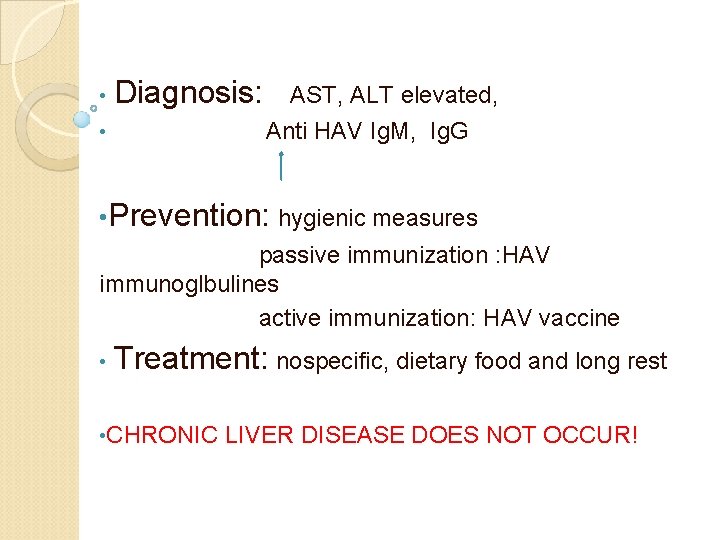
• Diagnosis: AST, ALT elevated, • Anti HAV Ig. M, Ig. G • Prevention: hygienic measures passive immunization : HAV immunoglbulines active immunization: HAV vaccine • Treatment: nospecific, dietary food and long rest • CHRONIC LIVER DISEASE DOES NOT OCCUR!

Hepatitis B virus

Hepatitis B Hepadnaviridae, DNA virus Transmission route is variable ◦ HBV is found in blood and all body fluids ◦ “Western” societies: percutaneous, hetero/homosexual contact is most common ◦ “Non-western” societies: perinatal transmission is most common Incubation: 1 -6 months During HBV infection, the host immune response causes both hepatocellular damage and viral clearance

Epidemiology *About 350 million people are chronically infected with HBV worldwide. *Despite the hepatitis B vaccine programs, new infections with HBV remain common. *Individuals with chronic hepatitis B are at increased risk for developing: a)cirrhosis, b)hepatic decompensation, c)hepatocellular carcinoma (HCC).


Clinical Presentation Acute Hepatitis B ; Based on significant aminotransferase activity due to necro inflammatory injury Symptoms are often non-specific symptoms such as myalgia, malaise , nausea, fatigue , pruritus, abdominal pain , jaundice Fulminant Hepatitis--Acute HBV results in Liver Failure Chronic Hepatitis B - greater than 6 months; Fibrosis and Necroinflammatory processes; can last for decades Immune tolerant phase: High viral replication, NL liver enzymes, low inflammation and fibrosis. Seen in children or those affected early in life. Immune active phase: High Liver enzymes and High HBV DNA and HBe. Ag, Active Replication ◦ Inactive Carrier State : Low HBV DNA levels, NL liver enzymes, Reduced Liver inflammation, Seroconversion from HBe. Ag to HBe. AB ◦ Low risk for developing of HCC

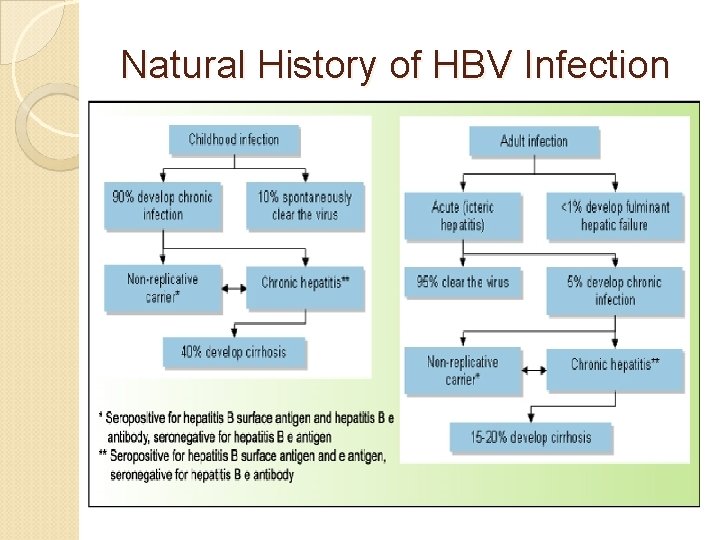
Natural History of HBV Infection

Acute Hepatitis B Virus Infection with Recovery. Typical Serologic Course Symptoms HBe. Ag anti-HBe Total anti-HBc Titre HBs. Ag 0 4 8 anti-HBs Ig. M anti-HBc 12 16 20 24 28 32 36 Weeks after Exposure 52 100

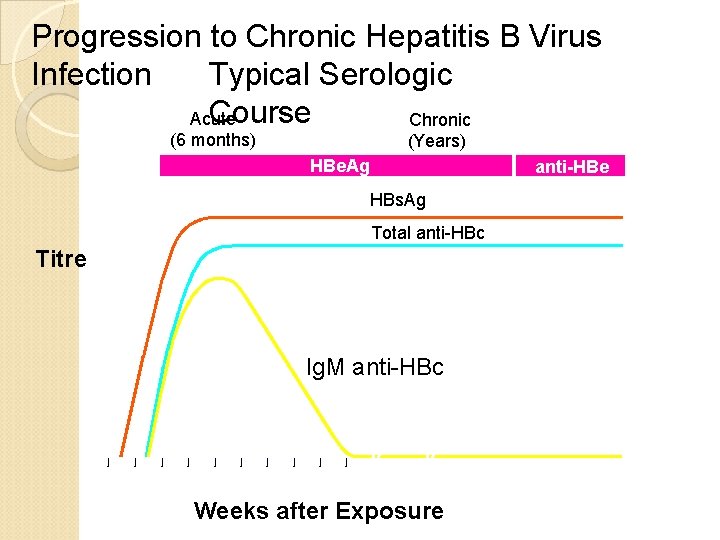
Progression to Chronic Hepatitis B Virus Typical Serologic Infection Course Acute Chronic (6 months) (Years)) HBe. Ag anti-HBe HBs. Ag Total anti-HBc Titre Ig. M anti-HBc 0 4 8 12 16 20 24 28 32 36 52 Weeks after Exposure Years


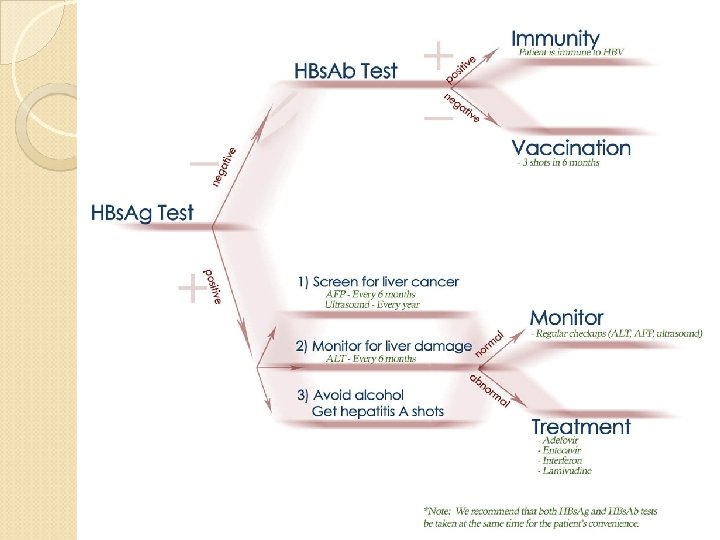
Hepatitis B Treatment Who to treat? ◦ HBs. Ag positive > 6 months ◦ HBV DNA >2000 IU/ml ◦ ALT elevated Goal of treatment ◦ Stop viral replication, HBV DNA becomes neg ◦ Convert HBe Ag pos to neg, Anti-HB e becomes pos ◦ Improvement in histology, prevention of progression to cirrhosis ◦ With successful treatment, loss of Surface Ag may occur in 1 -2% per year

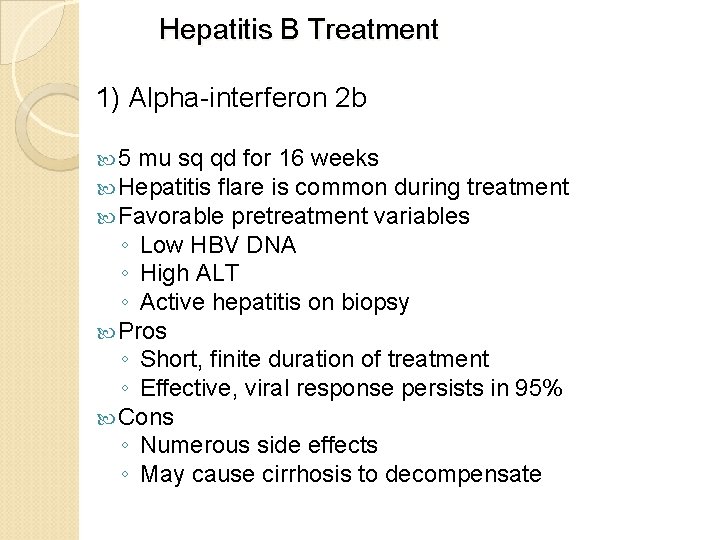
Hepatitis B Treatment 1) Alpha-interferon 2 b 5 mu sq qd for 16 weeks Hepatitis flare is common during treatment Favorable pretreatment variables ◦ Low HBV DNA ◦ High ALT ◦ Active hepatitis on biopsy Pros ◦ Short, finite duration of treatment ◦ Effective, viral response persists in 95% Cons ◦ Numerous side effects ◦ May cause cirrhosis to decompensate

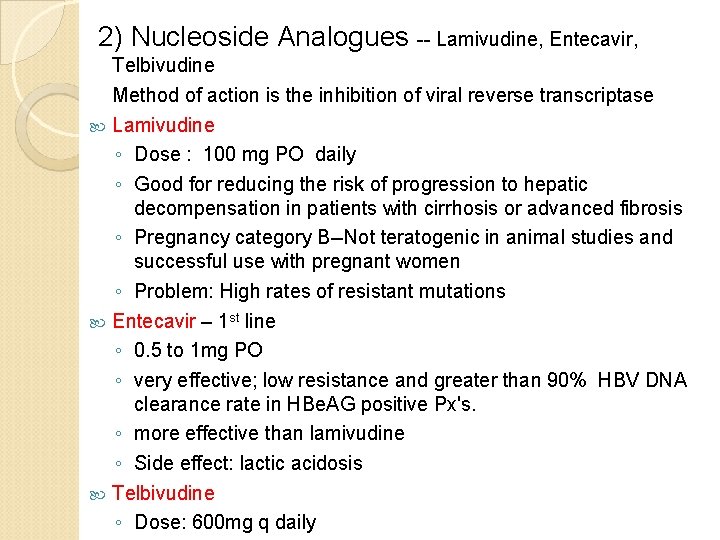
2) Nucleoside Analogues -- Lamivudine, Entecavir, Telbivudine Method of action is the inhibition of viral reverse transcriptase Lamivudine ◦ Dose : 100 mg PO daily ◦ Good for reducing the risk of progression to hepatic decompensation in patients with cirrhosis or advanced fibrosis ◦ Pregnancy category B--Not teratogenic in animal studies and successful use with pregnant women ◦ Problem: High rates of resistant mutations Entecavir – 1 st line ◦ 0. 5 to 1 mg PO ◦ very effective; low resistance and greater than 90% HBV DNA clearance rate in HBe. AG positive Px's. ◦ more effective than lamivudine ◦ Side effect: lactic acidosis Telbivudine ◦ Dose: 600 mg q daily

3)Nucleotide analogues: Tenfovir, Adefovir Method of action is the inhibition of viral reverse transcriptase Tenfovir ◦ Dose: 300 mg qd ◦ Highly effective with low resistance ◦ Well tolerated Adefovir Dose: 10 mg daily ◦ Side effect: nephrotoxicity and lactic acid Optimal treatment duration not yet defined


Prophylaxis HBV Vaccine Indicated for everyone and especially those in high risk groups ◦ IM injection at 0, 1, 6 months in infants and adults ◦ Response greater than 90% after 3 rd dose HBV Pregnant Mothers Give 1 st dose of Hip B vaccine and Hip B Immunoglobulin(HBIG) o. 5 ml within 12 hours of birth. ◦ 2 nd dose at 1 month, 3 rd at 6 months ◦ Recheck at 12 months for active infection ◦ 95% lifetime immunity ◦ Not Done---leads to 90% chronic HBV ◦ Transmitted through birth canal during birth or through umbilical cord. Others i. e. those receiving a needle stick Should receive 0. 04 to 0. 7 ml/kg of HBIG and 1 st dose vaccine within 48 and no later than a week

Hepatitis C virus

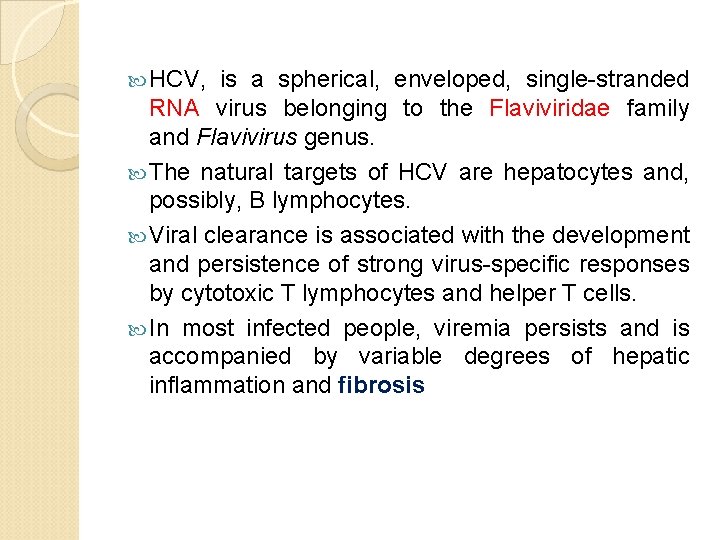
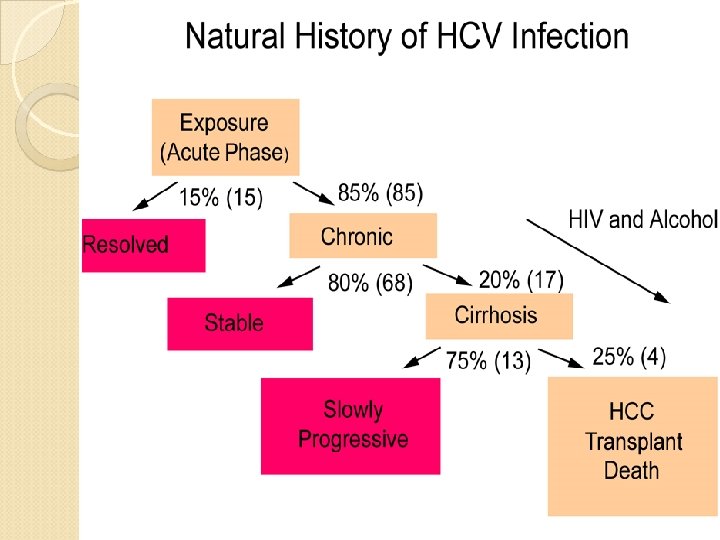
HCV, is a spherical, enveloped, single-stranded RNA virus belonging to the Flaviviridae family and Flavivirus genus. The natural targets of HCV are hepatocytes and, possibly, B lymphocytes. Viral clearance is associated with the development and persistence of strong virus-specific responses by cytotoxic T lymphocytes and helper T cells. In most infected people, viremia persists and is accompanied by variable degrees of hepatic inflammation and fibrosis

Epidemiology Hepatitis C is a worldwide problem. The WHO estimates that 170 million individuals worldwide are infected with HCV. Egypt had the highest number of reported infections, largely attributed to the use of contaminated parenteral antischistosomal therapy. This led to a mean prevalence of HCV antibodies in persons in Egypt of 22%.

Transmission Transfusion of contaminated blood. Persons who inject illegal drugs with non-sterile needles. Transmission of HCV to health care workers may occur via needle-stick injuries or other occupational exposures (3%). Tattooing, sharing razors, and acupuncture.

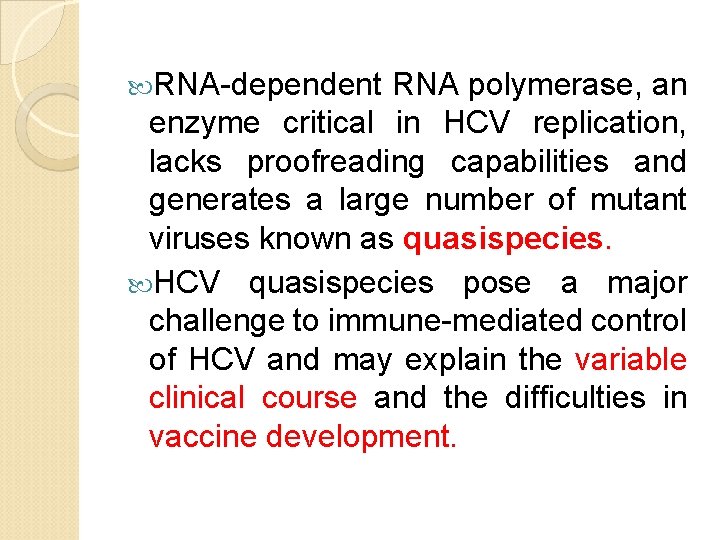
RNA-dependent RNA polymerase, an enzyme critical in HCV replication, lacks proofreading capabilities and generates a large number of mutant viruses known as quasispecies. HCV quasispecies pose a major challenge to immune-mediated control of HCV and may explain the variable clinical course and the difficulties in vaccine development.

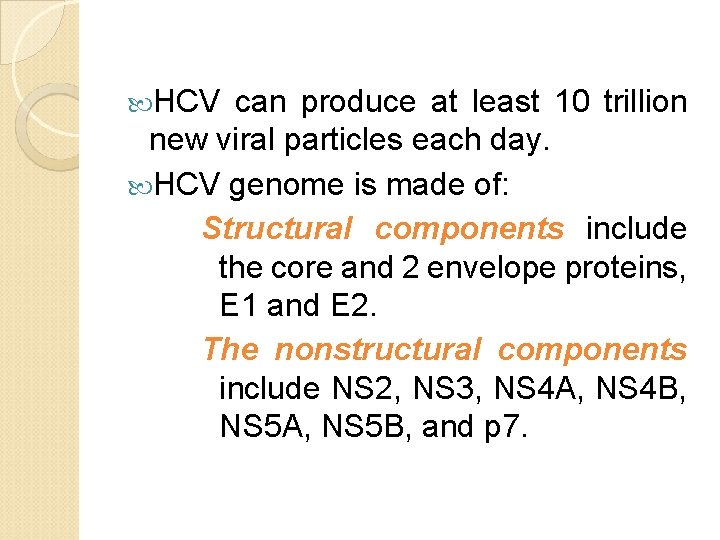
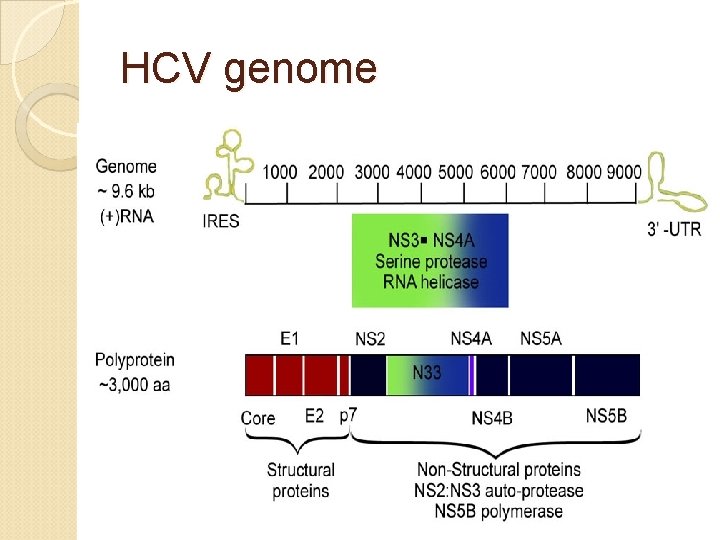
HCV can produce at least 10 trillion new viral particles each day. HCV genome is made of: Structural components include the core and 2 envelope proteins, E 1 and E 2. The nonstructural components include NS 2, NS 3, NS 4 A, NS 4 B, NS 5 A, NS 5 B, and p 7.

HCV genome

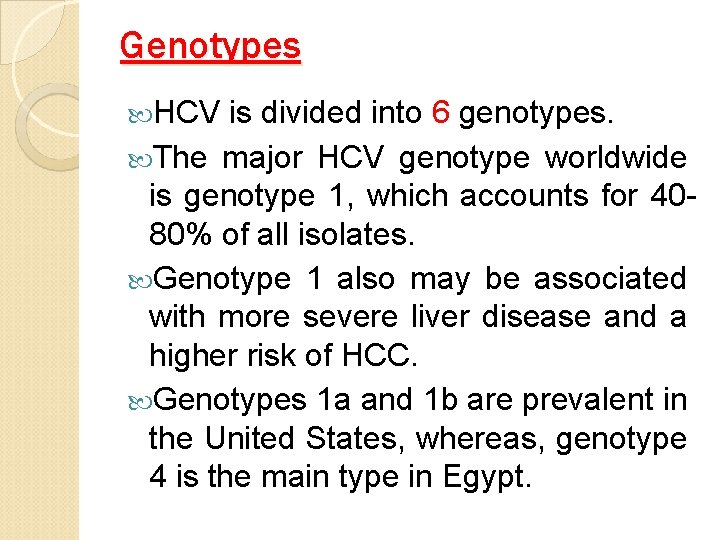
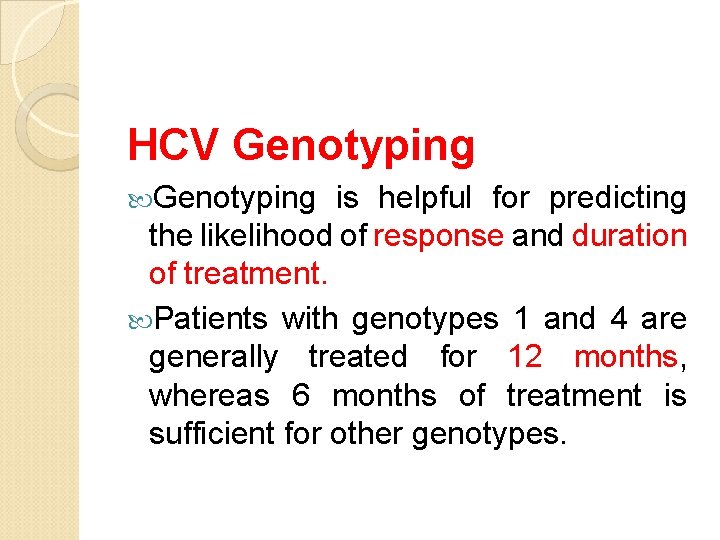
Genotypes HCV is divided into 6 genotypes. The major HCV genotype worldwide is genotype 1, which accounts for 4080% of all isolates. Genotype 1 also may be associated with more severe liver disease and a higher risk of HCC. Genotypes 1 a and 1 b are prevalent in the United States, whereas, genotype 4 is the main type in Egypt.


Clinical Presentation Asymptomatic Nonspecific symptoms Complications from advanced or decompensated liver disease Extrahepatic manifestations of HCV

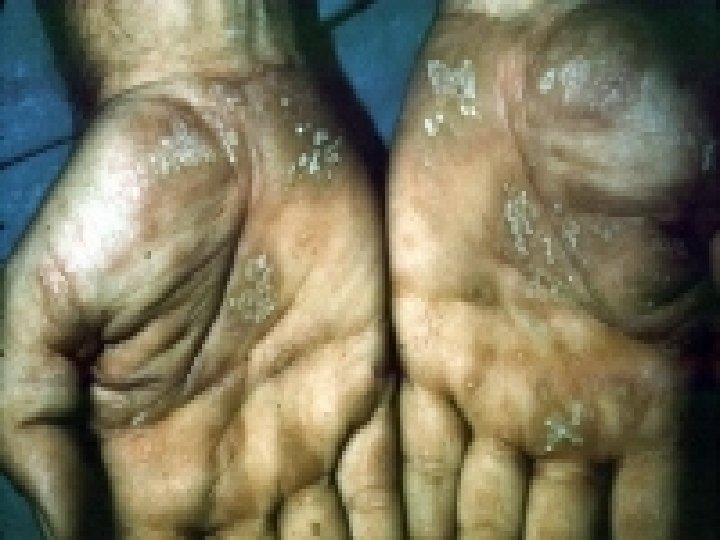
Extrahepatic Manifestations of Hepatitis C Membranoproliferative GN Essential Mixed Cryroglobulinemia Porphyria Cutanea Tarda Leukocytoclastic Vasculitis Mooren Corneal Ulcer Focal Lymphocytic Sialadenitits Lichen Planus Rheumatoid Arthritis Non-Hodgkin's Lymphoma Diabetes Mellitus +ANA 21%; +ASMA 21%; +ALKM 5%



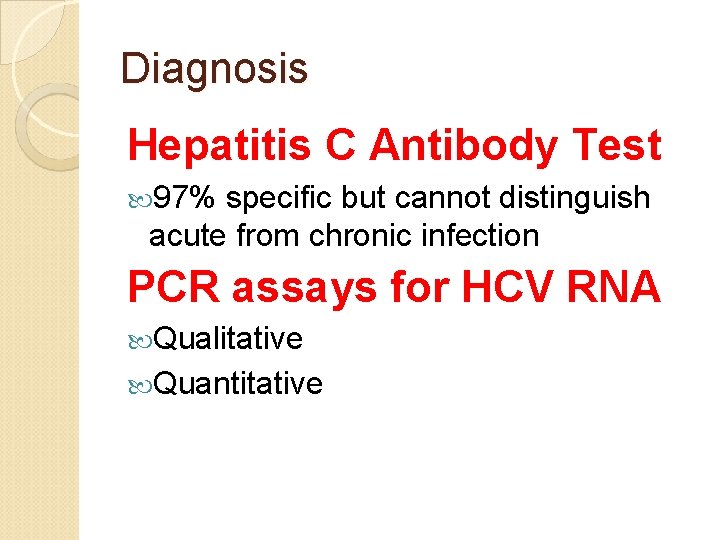
Diagnosis Hepatitis C Antibody Test 97% specific but cannot distinguish acute from chronic infection PCR assays for HCV RNA Qualitative Quantitative

HCV Genotyping is helpful for predicting the likelihood of response and duration of treatment. Patients with genotypes 1 and 4 are generally treated for 12 months, whereas 6 months of treatment is sufficient for other genotypes.


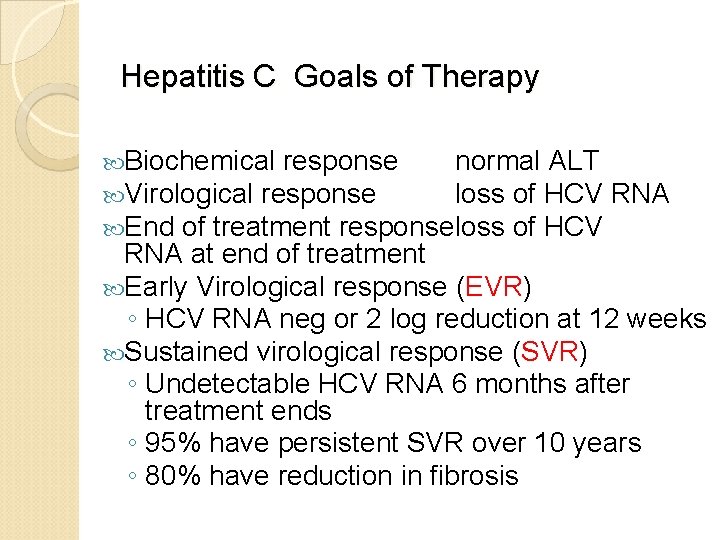
Hepatitis C Goals of Therapy Biochemical response normal ALT Virological response loss of HCV RNA End of treatment responseloss of HCV RNA at end of treatment Early Virological response (EVR) ◦ HCV RNA neg or 2 log reduction at 12 weeks Sustained virological response (SVR) ◦ Undetectable HCV RNA 6 months after treatment ends ◦ 95% have persistent SVR over 10 years ◦ 80% have reduction in fibrosis


What is Pegylation? Covalent attachment of polyethelene glycol to peptide Increases hydrodynamic size Prolonged circulation, delayed renal clearance Peg. Intron (12 kd, Schering), Pegasys (40 kd, Roche)

Hepatitis C Treatment: The Current Therapy The Future therapy (Direct-Acting Antivirals)

Current combined therapy Peg-interferon a-2 b (Peg Intron) 1. 5 ug/kg/wk sq Peg-interferon a-2 b (Peg Intron) ◦ 1. 5 ug/kg/wk sq Peg-interferon a-2 a (Pegasys) ◦ 180 ug/wk sq Ribavirin ◦ 1000 -1200 mg/d for genotype 1 ◦ 800 mg/d for genotype non-1 Treat genotype 1: 48 weeks; genotype non-1: 24 weeks

Side Effects of Peg. IFN • • Depression ranging from mild to suicidality Irritability, aggressive behavior Worsening of mania Fatigue Insomnia Myalgias, fever, flu-like symptoms Hair loss Cytopenias

Side Effects of Ribavirin ◦ Hemolytic anemia Relatively contraindicated with CAD, advanced age, renal insufficiency Improves with dose reduction or erythropoietin ◦ Headache ◦ Teratogenic Women must be infertile or on OCP Men cannot conceive on therapy and 6 months off therapy

The Future (Direct-Acting Antivirals) 1 -Protease Inhibitors Boceprevir (Victrelis) Telaprevir (Incivek) Simeprevir (Olysio) Triple Therapy PEG-Interferon/Ribavirin/Protease Inhibitors

2 -Nucleotide HCV polymerase (NS 5 B) inhibitor: Sofosbuvir (Sovaldi) Once a day dosing All genotypes treated Minimal side effects/drug interactions Sofosbuvir + Ribavirin: 1 st Interferon-free treatment 24 wks 12 wks Sofosbuvir/Riba/PEG-IFN 3 -Sofosbuvir + Ledipasvir; one pill a day; (Harvoni)

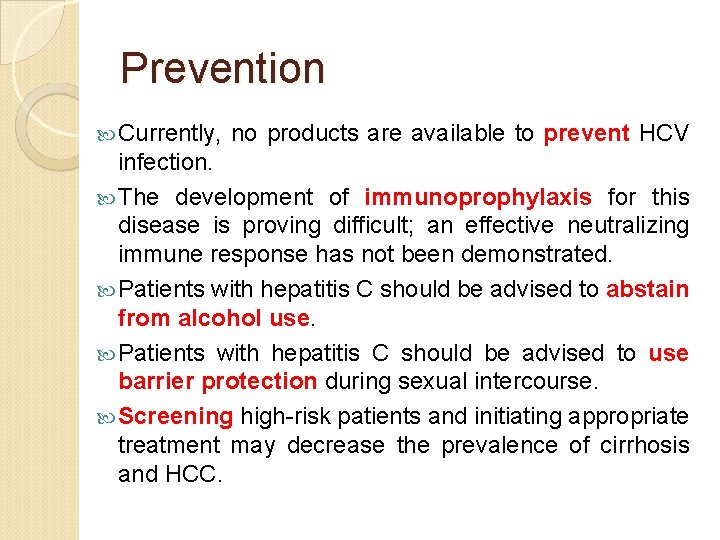
Prevention Currently, no products are available to prevent HCV infection. The development of immunoprophylaxis for this disease is proving difficult; an effective neutralizing immune response has not been demonstrated. Patients with hepatitis C should be advised to abstain from alcohol use. Patients with hepatitis C should be advised to use barrier protection during sexual intercourse. Screening high-risk patients and initiating appropriate treatment may decrease the prevalence of cirrhosis and HCC.

Hepatitis D (Delta) Virus

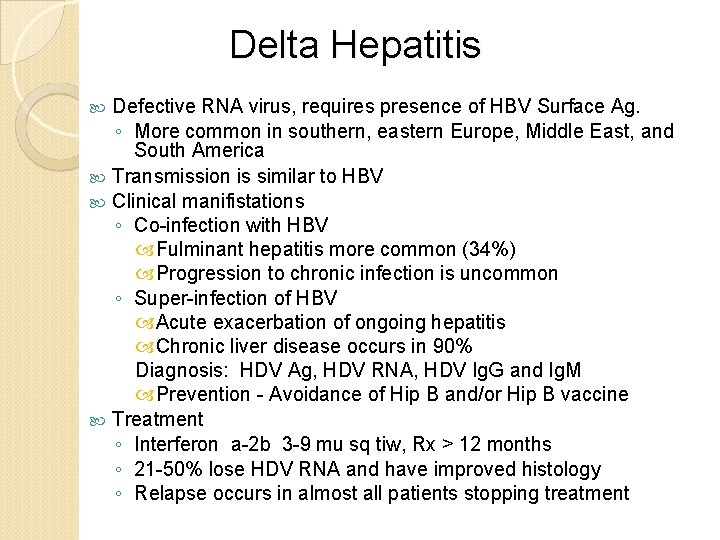
Delta Hepatitis Defective RNA virus, requires presence of HBV Surface Ag. ◦ More common in southern, eastern Europe, Middle East, and South America Transmission is similar to HBV Clinical manifistations ◦ Co-infection with HBV Fulminant hepatitis more common (34%) Progression to chronic infection is uncommon ◦ Super-infection of HBV Acute exacerbation of ongoing hepatitis Chronic liver disease occurs in 90% Diagnosis: HDV Ag, HDV RNA, HDV Ig. G and Ig. M Prevention - Avoidance of Hip B and/or Hip B vaccine Treatment ◦ Interferon a-2 b 3 -9 mu sq tiw, Rx > 12 months ◦ 21 -50% lose HDV RNA and have improved histology ◦ Relapse occurs in almost all patients stopping treatment

Hepatitis E virus (HEV) RNA virus of the genus Hepevirus Enterically transmitted infection; fecal-oral route, typically self-limited Most outbreaks occur in developing countries. Symptoms of acute hepatitis Incubation period of hepatitis E virus is 2 -9 weeks 1 -4% overall mortality; 20 -30% mortality if pregnant

Hepatitis E: diagnosis Serum, liver, and stool samples can be tested for HEV RNA Anti-HEV antibodies: - Ig. M (acute) - Ig. G (chronic) AST & ALT are elevated several days before the onset of symptoms; return to normal within 1 -2 months after the peak severity of disease. Treatment: supportive

 Saharzakaria
Saharzakaria Hepatomogaly
Hepatomogaly Zakaria chowdhury
Zakaria chowdhury Ethnography synonym
Ethnography synonym Fareed zakaria american dream
Fareed zakaria american dream Zakaria abdulla
Zakaria abdulla Ober's test
Ober's test Sahar mahdi
Sahar mahdi Umt library catalogue
Umt library catalogue Sahar mosleh
Sahar mosleh Sahar mahdi
Sahar mahdi Sahar mosleh
Sahar mosleh Maturation and development
Maturation and development Sahar mosleh
Sahar mosleh Sahar mahdi
Sahar mahdi Uterine atony
Uterine atony Sahar mahdi
Sahar mahdi Sahar mosleh
Sahar mosleh Sahar anwar
Sahar anwar Sahar mosleh
Sahar mosleh Kristine kousholt
Kristine kousholt Sahar mahdi
Sahar mahdi Sahar zia
Sahar zia Sahar mosleh
Sahar mosleh Infectious canine hepatitis in dogs
Infectious canine hepatitis in dogs Hepatitis c symtoms
Hepatitis c symtoms Gail lupica
Gail lupica Papillomitosis
Papillomitosis Colestasis gestacional
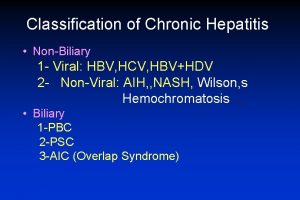
Colestasis gestacional Classification of chronic hepatitis
Classification of chronic hepatitis Hepatitis alimentos permitidos y no permitidos
Hepatitis alimentos permitidos y no permitidos Pictures of body lice and scabies
Pictures of body lice and scabies Alcoholic hepatitis
Alcoholic hepatitis Klasifikasi hepatitis a
Klasifikasi hepatitis a Hep c symptoms
Hep c symptoms Hepatitis b panel
Hepatitis b panel Ligamentum liver
Ligamentum liver Hepatitis types table
Hepatitis types table Ano ang pagkakaiba ng hepatitis a at b
Ano ang pagkakaiba ng hepatitis a at b Hepatitis c symptoms
Hepatitis c symptoms Grados de encefalopatia hepatica
Grados de encefalopatia hepatica Hepatitis c symptoms in men
Hepatitis c symptoms in men Std refers to *
Std refers to * Types of cirrhosis
Types of cirrhosis Michael le md
Michael le md Esophageal varices nursing management
Esophageal varices nursing management Cholecystitis nursing care plan
Cholecystitis nursing care plan Chronic hepatitis
Chronic hepatitis Colestasis
Colestasis Hepatitis e
Hepatitis e Hepatitis
Hepatitis Hepatitis types chart
Hepatitis types chart Porta hepatis
Porta hepatis Infeccion por vph
Infeccion por vph Hepatitis b vaccine schedule for adults
Hepatitis b vaccine schedule for adults Phases of chronic hepatitis b
Phases of chronic hepatitis b Score simplificado hepatitis autoinmune
Score simplificado hepatitis autoinmune