Hepatitis C in Primary Care Mary Alice Walker




















- Slides: 40

Hepatitis C in Primary Care Mary Alice Walker, FNP Family Nurse Practitioner, Penobscot Community Health Care Kathryn Cunniff, Pharm. D Primary Care Pharmacist, Penobscot Community Health Care

Disclosures The following personal financial relationships with commercial interests relevant to this presentation existed during the past 12 months: Mary Alice Walker: No relationships to disclose Kathryn Cunniff: No relationships to disclose

Session Objectives Recognize ways to increase hepatitis C screening Formulate a workflow to schedule, assess, and treat patients Introduce key resources and review medications Practice a patient case Review data and outcomes at PCHC

Overview of Penobscot Community Health Care (PCHC) � Established in 1998 � Federally Qualified Health Center � 16 practice sites � 100 Primary Care Providers � 40 Psychiatric Providers � 18 Pharmacists � 13 Dentists � 7 Physical Therapists � 3 Chiropractors � In 2017, 65, 000+ patients � Majority uninsured or underinsured � 4 integrated pharmacies

Increasing Hepatitis C Screening The CDC’s 2020 goal for hepatitis C is to reduce the rate of acute HCV infections to 0. 25 cases per 100, 000 U. S. population.

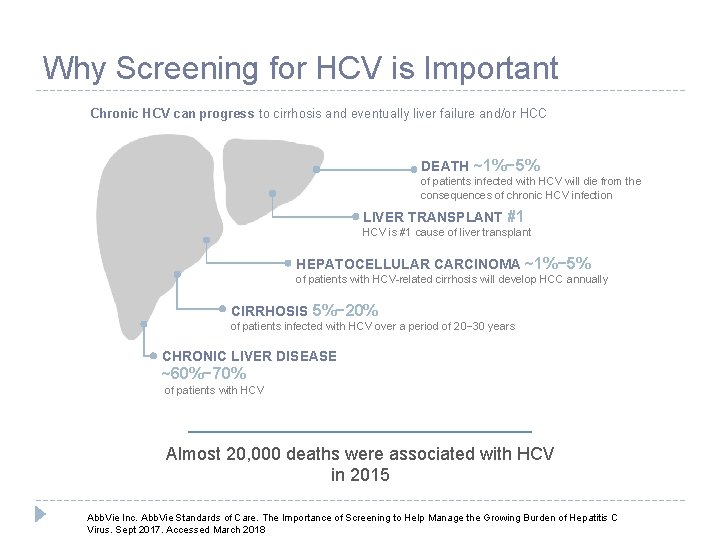
Why Screening for HCV is Important Chronic HCV can progress to cirrhosis and eventually liver failure and/or HCC DEATH ~1%− 5% of patients infected with HCV will die from the consequences of chronic HCV infection LIVER TRANSPLANT #1 HCV is #1 cause of liver transplant HEPATOCELLULAR CARCINOMA ~1%− 5% of patients with HCV-related cirrhosis will develop HCC annually CIRRHOSIS 5%− 20% of patients infected with HCV over a period of 20− 30 years CHRONIC LIVER DISEASE ~60%− 70% of patients with HCV Almost 20, 000 deaths were associated with HCV in 2015 6 Abb. Vie Inc. Abb. Vie Standards of Care. The Importance of Screening to Help Manage the Growing Burden of Hepatitis C Virus. Sept 2017. Accessed March 2018

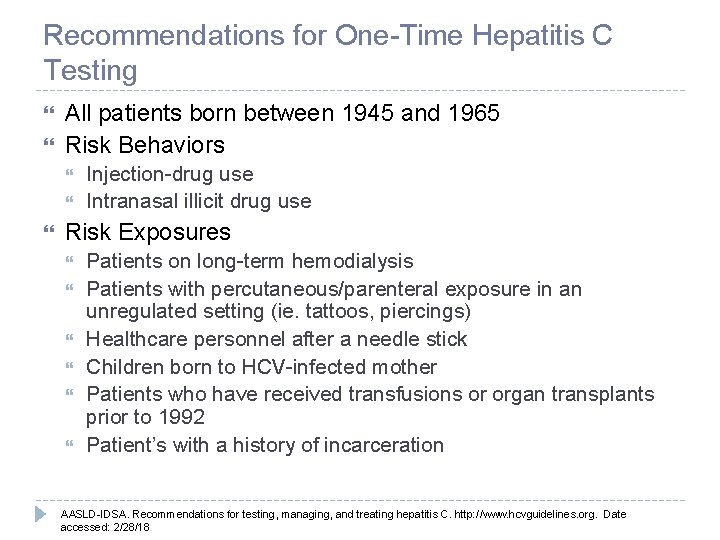
Recommendations for One-Time Hepatitis C Testing All patients born between 1945 and 1965 Risk Behaviors Injection-drug use Intranasal illicit drug use Risk Exposures Patients on long-term hemodialysis Patients with percutaneous/parenteral exposure in an unregulated setting (ie. tattoos, piercings) Healthcare personnel after a needle stick Children born to HCV-infected mother Patients who have received transfusions or organ transplants prior to 1992 Patient’s with a history of incarceration AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. http: //www. hcvguidelines. org. Date accessed: 2/28/18

Recommendations for One-Time Hepatitis C Testing Other Conditions and Circumstances HIV infection Sexually active persons about to start Pre. P for HIV Unexplained chronic liver disease and/or chronic hepatitis, including elevated ALT levels Solid organ donors AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. http: //www. hcvguidelines. org. Date accessed: 2/28/18

Recommendation for HCV Testing for Persons with Ongoing Risk Factors Annual HCV testing is recommended for persons who Inject drugs HIV-infected men who have unprotected sex with men Periodic testing should be offered to any patients with ongoing risk factors for HCV exposure AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. http: //www. hcvguidelines. org. Date accessed: 2/28/18

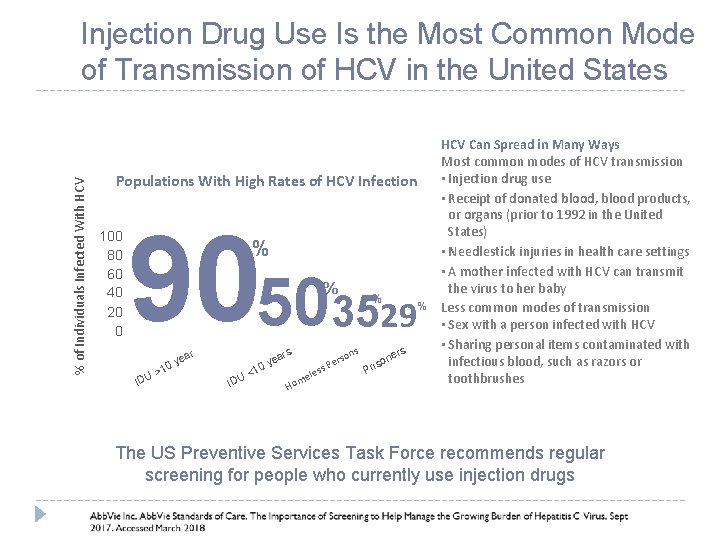
% of Individuals Infected With HCV Injection Drug Use Is the Most Common Mode of Transmission of HCV in the United States Populations With High Rates of HCV Infection 100 80 60 40 20 0 9050 % % rs r IDU >1 ea 0 y IDU <1 ea 0 y ons rs Pe s s e el m Ho 3529 % ers P n riso % HCV Can Spread in Many Ways Most common modes of HCV transmission • Injection drug use • Receipt of donated blood, blood products, or organs (prior to 1992 in the United States) • Needlestick injuries in health care settings • A mother infected with HCV can transmit the virus to her baby Less common modes of transmission • Sex with a person infected with HCV • Sharing personal items contaminated with infectious blood, such as razors or toothbrushes The US Preventive Services Task Force recommends regular screening for people who currently use injection drugs 10

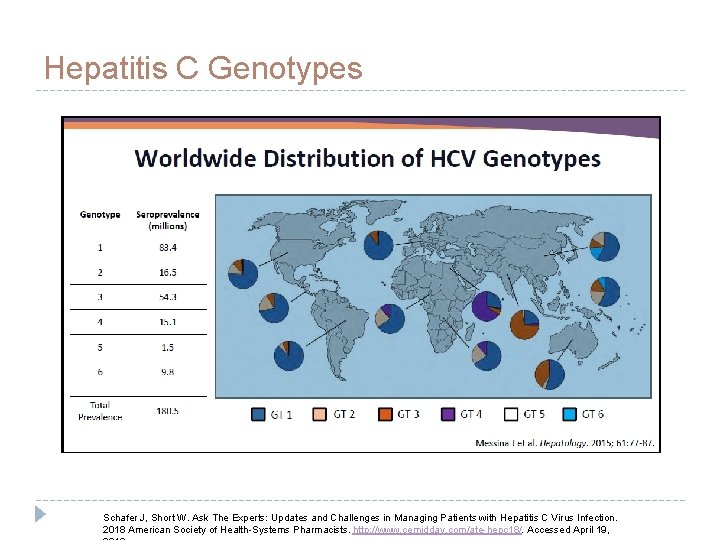
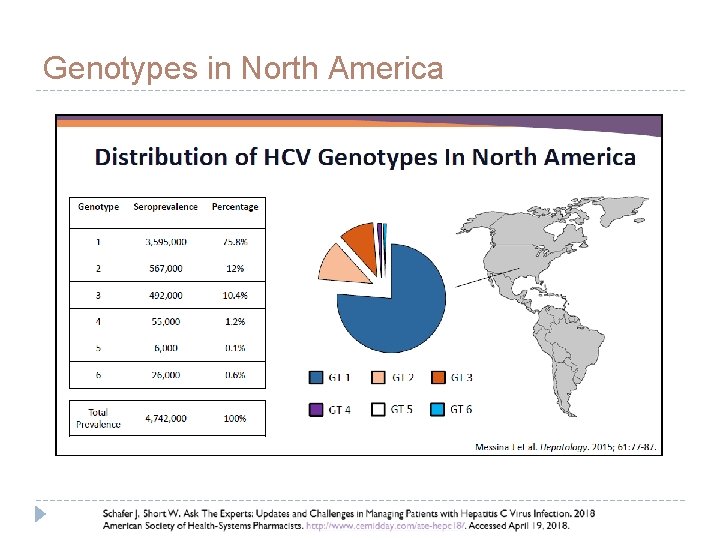
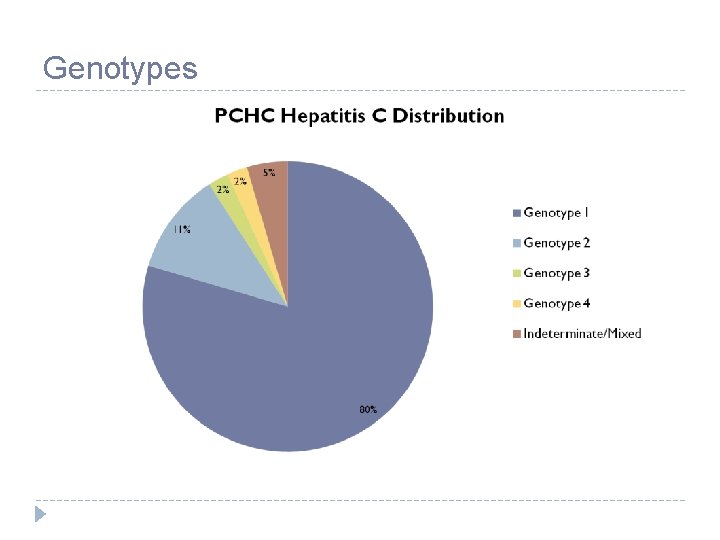
Hepatitis C Genotypes Schafer J, Short W. Ask The Experts: Updates and Challenges in Managing Patients with Hepatitis C Virus Infection. 2018 American Society of Health-Systems Pharmacists. http: //www. cemidday. com/ate-hepc 18/. Accessed April 19,

Genotypes in North America

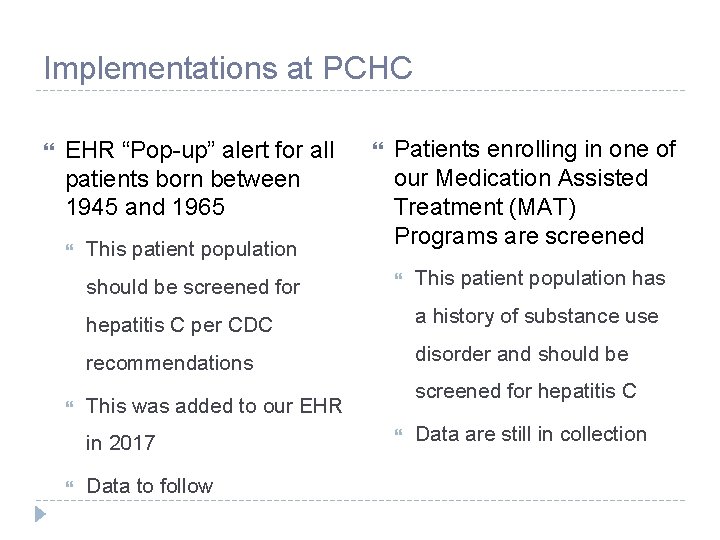
Implementations at PCHC EHR “Pop-up” alert for all patients born between 1945 and 1965 This patient population should be screened for Patients enrolling in one of our Medication Assisted Treatment (MAT) Programs are screened This patient population has hepatitis C per CDC a history of substance use recommendations disorder and should be screened for hepatitis C This was added to our EHR in 2017 Data to follow Data are still in collection

Formulate a Workflow to Connect Patients to Treatment Review resources and medications

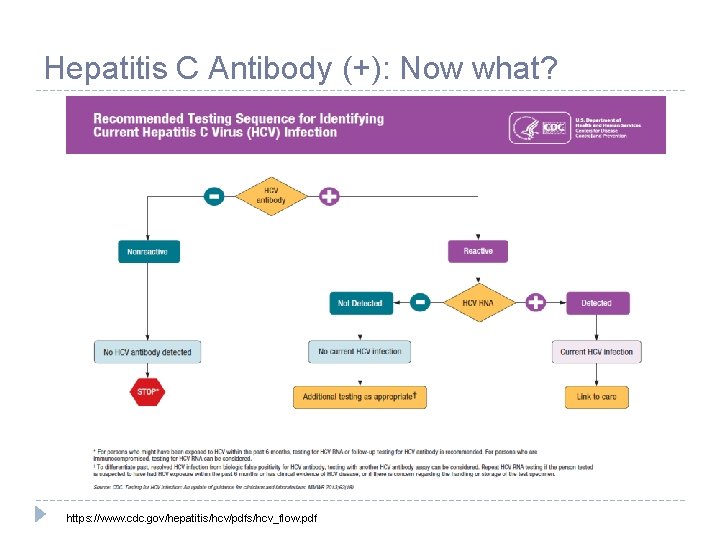
Hepatitis C Antibody (+): Now what? https: //www. cdc. gov/hepatitis/hcv/pdfs/hcv_flow. pdf

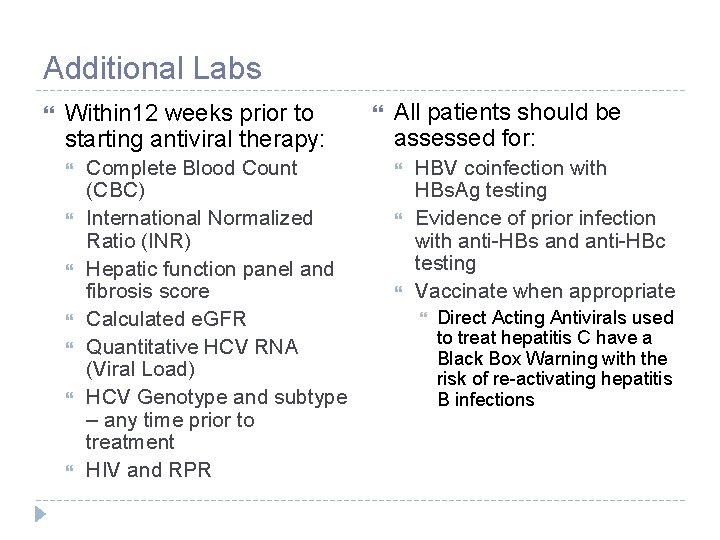
Additional Labs Within 12 weeks prior to starting antiviral therapy: Complete Blood Count (CBC) International Normalized Ratio (INR) Hepatic function panel and fibrosis score Calculated e. GFR Quantitative HCV RNA (Viral Load) HCV Genotype and subtype – any time prior to treatment HIV and RPR All patients should be assessed for: HBV coinfection with HBs. Ag testing Evidence of prior infection with anti-HBs and anti-HBc testing Vaccinate when appropriate Direct Acting Antivirals used to treat hepatitis C have a Black Box Warning with the risk of re-activating hepatitis B infections

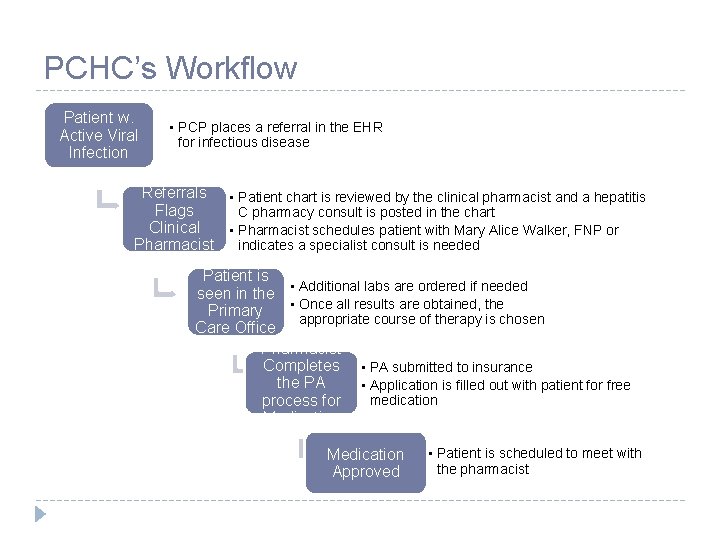
PCHC’s Workflow Patient w. Active Viral Infection • PCP places a referral in the EHR for infectious disease Referrals • Patient chart is reviewed by the clinical pharmacist and a hepatitis Flags C pharmacy consult is posted in the chart Clinical • Pharmacist schedules patient with Mary Alice Walker, FNP or indicates a specialist consult is needed Pharmacist Patient is seen in the • Additional labs are ordered if needed • Once all results are obtained, the Primary appropriate course of therapy is chosen Care Office Pharmacist Completes • PA submitted to insurance the PA • Application is filled out with patient for free medication process for Medication Approved • Patient is scheduled to meet with the pharmacist

Monitoring Labs After 4 Weeks of Treatment CBC Creatinine level Calculated glomerular filtration rate (e. GFR) Hepatic Function Panel Quantitative HCV viral load

Pharmacist’s Role Initial consult at first medication fill Phone check-in at weeks 2, 4, 8, and 12 if indicated Review of prescription and OTC medication use Common side effects and management strategies Encourage adherence to regimen, and identify potential barriers Assess adherence and any adverse reactions Schedule patient for follow up labs Coordinate medication refills Track outcomes once patient has completed therapy

Resources Clinical Sources AASLD and IDSA Guidelines Centers for Disease Control and Prevention (CDC) Project ECHO Nova. Med Education – HCV Clinician Toolbox Fibrosis 4 (FIB 4) Score Calculator Pharmacy Sources University of Liverpool – HEP Drug Interactions Cover. My. Meds Gilead’s Support Path Program

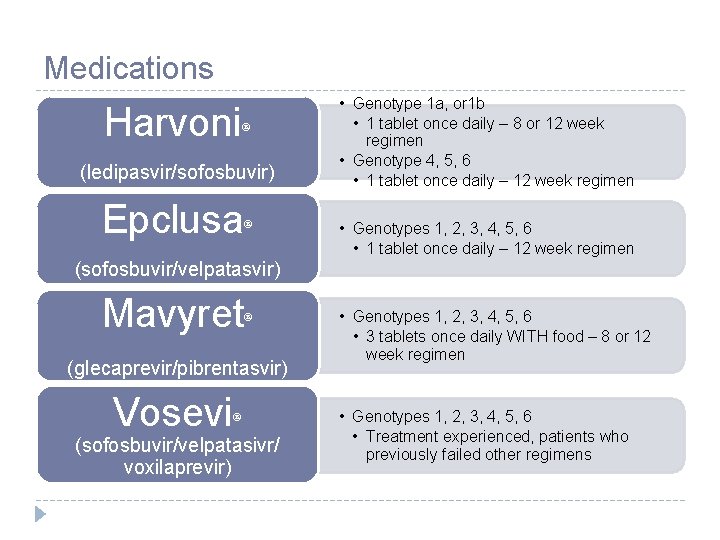
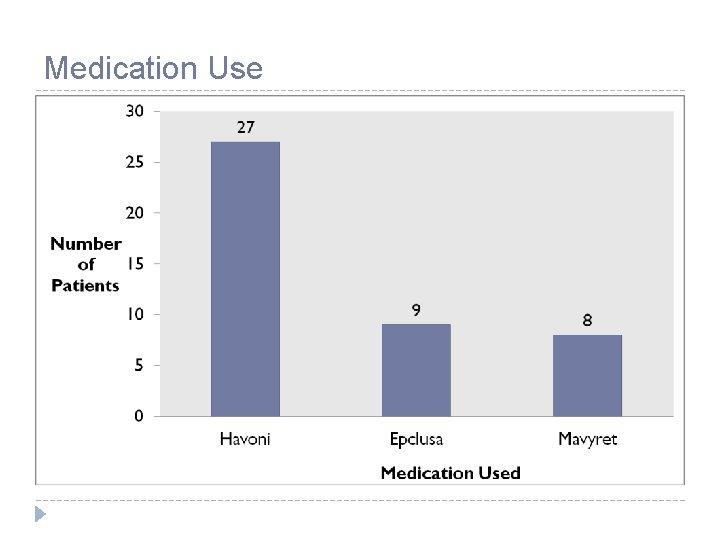
Medications Harvoni (ledipasvir/sofosbuvir) Epclusa (sofosbuvir/velpatasvir) Mavyret (glecaprevir/pibrentasvir) Vosevi (sofosbuvir/velpatasivr/ voxilaprevir) • Genotype 1 a, or 1 b • 1 tablet once daily – 8 or 12 week regimen • Genotype 4, 5, 6 • 1 tablet once daily – 12 week regimen • Genotypes 1, 2, 3, 4, 5, 6 • 1 tablet once daily – 12 week regimen • Genotypes 1, 2, 3, 4, 5, 6 • 3 tablets once daily WITH food – 8 or 12 week regimen • Genotypes 1, 2, 3, 4, 5, 6 • Treatment experienced, patients who previously failed other regimens

Patient Case Work together to solve the following case

Patient: JM 34 year old white male Patient tested positive for hepatitis C in November of 2015. Patient has never been treated for hepatitis C Patient medications: 2010 through 2011: Patient used IV and intranasal drugs for approximately one year 2012: patient tested negative for hepatitis C 2015 patient had a tattoo done on right leg ranitidine 150 mg twice daily Suboxone 8 -2 mg film daily What counseling should be provided to this patient? What labs are indicated for this patient?

Counseling & Lab Orders Patient counseling Labs Discuss ways to reduce liver disease progression and how to prevent transmission Encourage abstinence from alcohol and offer support if needed HIV co-infection Hepatitis A and B history CBC INR Hepatic function panel Calculated e. GFR Quantitative HCV RNA (viral load) HCV genotype and subtype Staging of hepatic fibrosis (ie. FIB-4, or liver elastography) Additionally: Vaccinate for Hep A and B if patient is not immune Vaccinate against pneumococcal infection in cirrhotic patients

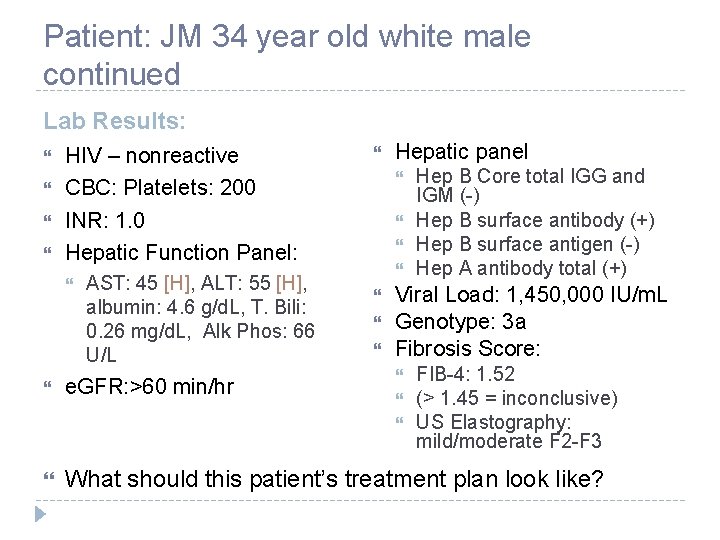
Patient: JM 34 year old white male continued Lab Results: HIV – nonreactive CBC: Platelets: 200 INR: 1. 0 Hepatic Function Panel: AST: 45 [H], ALT: 55 [H], albumin: 4. 6 g/d. L, T. Bili: 0. 26 mg/d. L, Alk Phos: 66 U/L e. GFR: >60 min/hr Hepatic panel Viral Load: 1, 450, 000 IU/m. L Genotype: 3 a Fibrosis Score: Hep B Core total IGG and IGM (-) Hep B surface antibody (+) Hep B surface antigen (-) Hep A antibody total (+) FIB-4: 1. 52 (> 1. 45 = inconclusive) US Elastography: mild/moderate F 2 -F 3 What should this patient’s treatment plan look like?

Determining a Treatment Plan Choose medication Determine length of treatment Identify potential drug interactions Create a plan to have frequent contact with the patient After 4 weeks of therapy: i. e. phone calls or clinic visits Monitor labs: CBC, SCr, e. GFR, hepatic function, and viral load Additional viral load testing Consider at end of treatment To ensure cure draw 12 weeks following completion of therapy

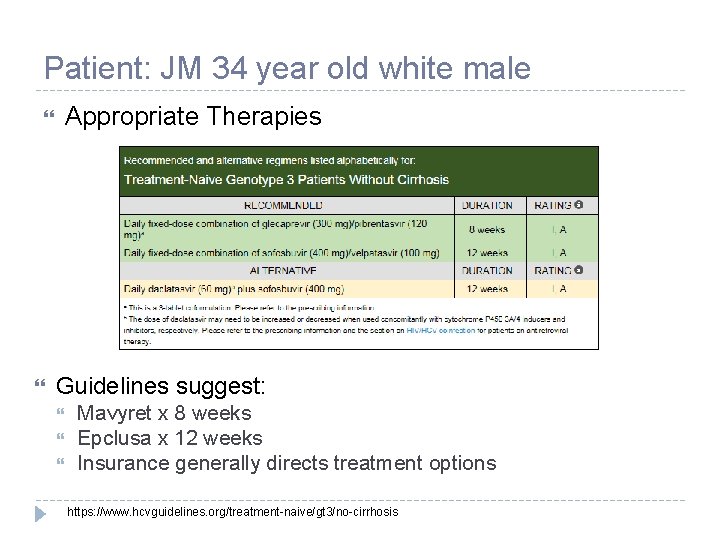
Patient: JM 34 year old white male Appropriate Therapies Guidelines suggest: Mavyret x 8 weeks Epclusa x 12 weeks Insurance generally directs treatment options https: //www. hcvguidelines. org/treatment-naive/gt 3/no-cirrhosis

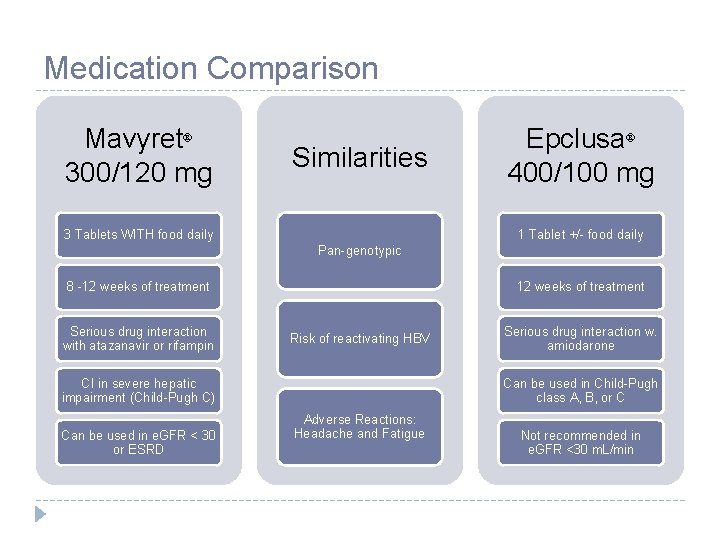
Medication Comparison Mavyret 300/120 mg Similarities 3 Tablets WITH food daily Epclusa 400/100 mg 1 Tablet +/- food daily Pan-genotypic 8 -12 weeks of treatment Serious drug interaction with atazanavir or rifampin 12 weeks of treatment Risk of reactivating HBV CI in severe hepatic impairment (Child-Pugh C) Can be used in e. GFR < 30 or ESRD Serious drug interaction w. amiodarone Can be used in Child-Pugh class A, B, or C Adverse Reactions: Headache and Fatigue Not recommended in e. GFR <30 m. L/min

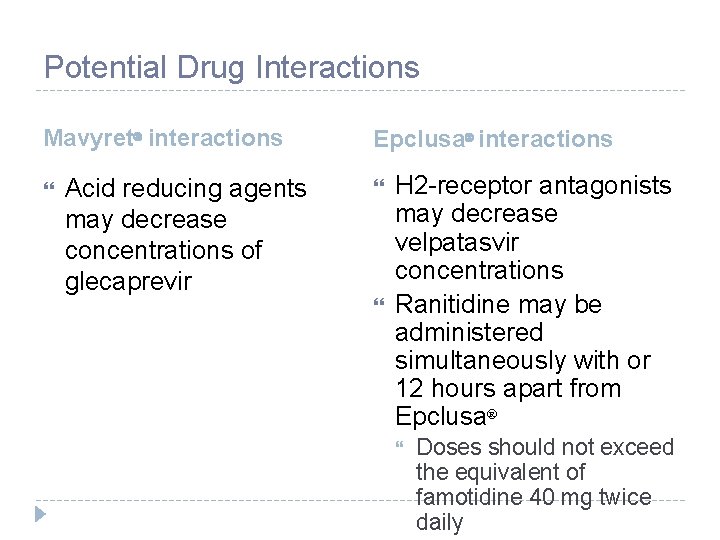
Potential Drug Interactions Mavyret interactions Acid reducing agents may decrease concentrations of glecaprevir Epclusa interactions H 2 -receptor antagonists may decrease velpatasvir concentrations Ranitidine may be administered simultaneously with or 12 hours apart from Epclusa Doses should not exceed the equivalent of famotidine 40 mg twice daily

Patient Monitoring Plan Check-ins and Monitoring Pharmacist calls after 2 weeks of treatment Patient is scheduled for a clinic visit after 4 weeks of treatment Labs drawn Pharmacist calls when each refill is ready Patient is scheduled for a clinic visit upon treatment completion Reminded of future labs Schedule labs for 4 weeks, end of treatment, and 12 weeks following treatment Cure = Sustained virologic response (SVR) 12 weeks following final dose of medication

Penobscot Community Health Care Data and Outcomes

PCHC’s Hepatitis C Clinic at Brewer Medical Center Since integrating hepatitis C treatment into our primary care setting in 2017 We have treated 38 patients 14 of which we have SVR results All are considered cured of hepatitis C We currently have 11 patients on treatment As we continue to grow our program we hope to reach more patients in our community

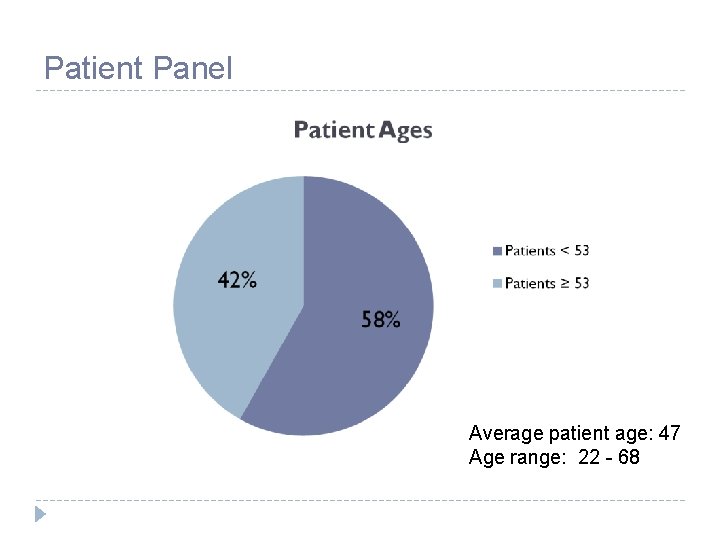
Patient Panel Average patient age: 47 Age range: 22 - 68

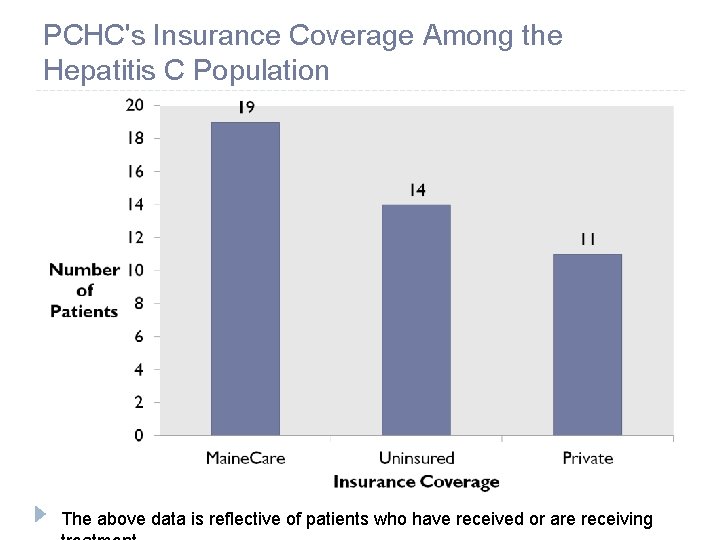
PCHC's Insurance Coverage Among the Hepatitis C Population The above data is reflective of patients who have received or are receiving

Genotypes

Medication Use

Barriers to Treatment Uninsured High cost of labs High cost of medications Application available through the pharmaceutical companies Lost to follow up Unreliable means of communication and transportation Multiple co-morbidities Mental health Substance use disorder Hepatic complications

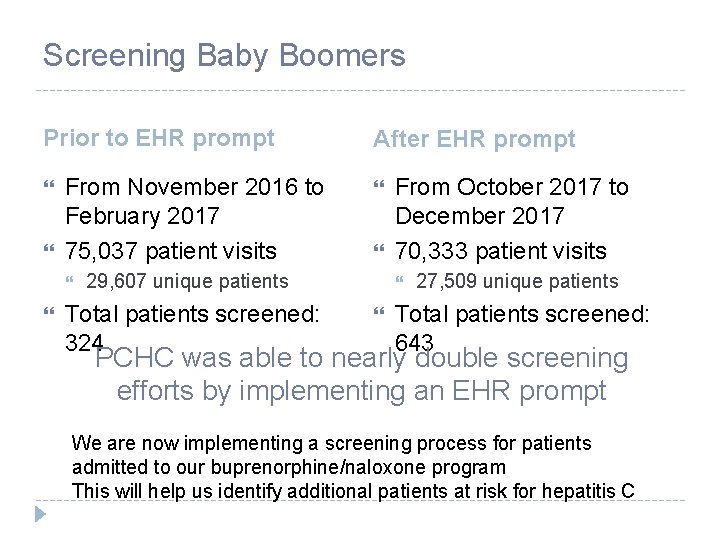
Screening Baby Boomers Prior to EHR prompt From November 2016 to February 2017 75, 037 patient visits After EHR prompt 29, 607 unique patients Total patients screened: 324 From October 2017 to December 2017 70, 333 patient visits 27, 509 unique patients Total patients screened: 643 PCHC was able to nearly double screening efforts by implementing an EHR prompt We are now implementing a screening process for patients admitted to our buprenorphine/naloxone program This will help us identify additional patients at risk for hepatitis C

Questions?

References: CDC. Testing for HCV infection: An update of guidance for clinicians and laboratorians. MMWR 2013; 62(18). CDC. Hepatitis FAQs for health professionals. https: //www. cdc. gov/hepatitis/hcvfaq. htm. Updated January 27, 2017. Mutimer D et al. J Hepatol. 2014; 60(2): 392‐ 420. CDC. Surveillance for viral hepatitis—United States, 2015. https: //www. cdc. gov/hepatitis/statistics/2015 surveillance/commentary. htm. Updated June 19, 2017. 1. CDC. Hepatitis C FAQs for health professionals. https: //www. cdc. gov/hepatitis/hcvfaq. htm. Updated January 27, 2017. 2. Edlin BR. Nature. 2011; 474(7350): S 18 -S 19. 3. US Preventive Services Task Force. Understanding Task Force Recommendations: Screening for hepatitis C virus infection in adults. https: //www. uspreventiveservicestaskforce. org/Home/Get. File. By. ID/1888. Published June 2013 AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C. http: //www. hcvguidelines. org. Accessed March 7, 2018. Harvoni [package insert]. Foster City, CA. Gilead Sciences, Inc. 2017. Epclusa [package insert]. Foster City, CA. Gilead Sciences, Inc. 2017. Mavyret [package insert]. North Chicago, IL. Abb. Vie Inc. 2017. Vosevi [package insert]. Foster City, CA. Gilead Sciences Inc. 2017