Hepatic stellate cells as key target in liver




- Slides: 12

Hepatic stellate cells as key target in liver fibrosis 2017. 12. 08

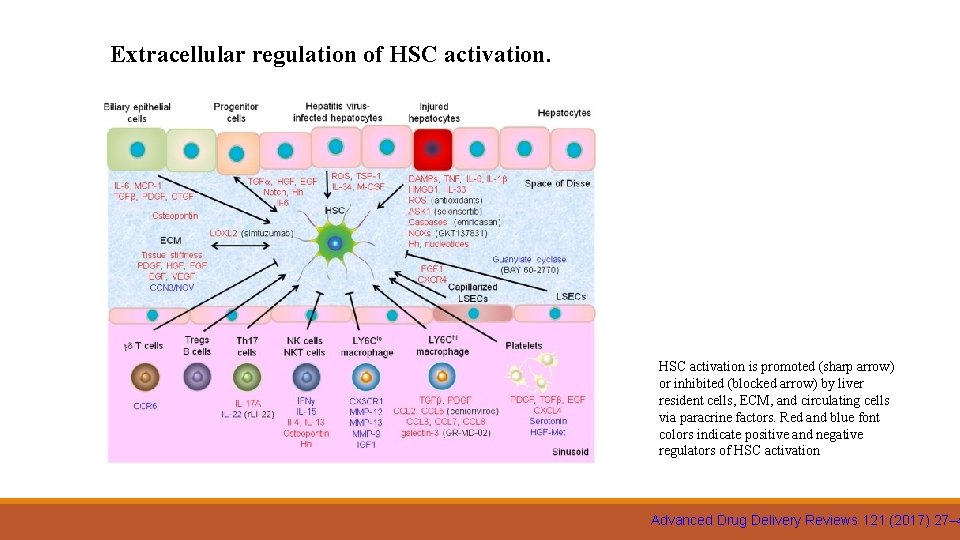
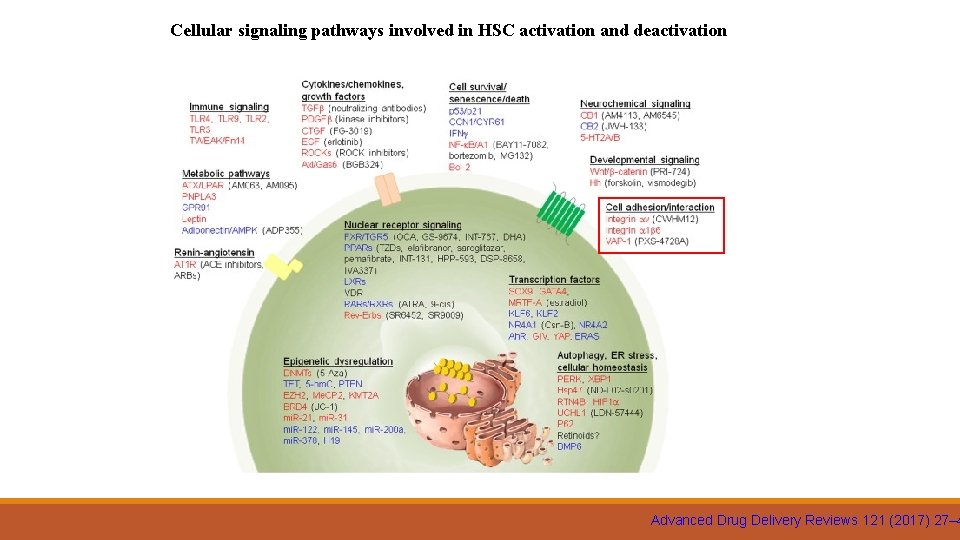
Extracellular regulation of HSC activation is promoted (sharp arrow) or inhibited (blocked arrow) by liver resident cells, ECM, and circulating cells via paracrine factors. Red and blue font colors indicate positive and negative regulators of HSC activation Advanced Drug Delivery Reviews 121 (2017) 27– 4

Pathways regulating HSC activation Fibrogenic and proliferative cytokines § § - TGFb smad 3 phospholylation promotes collagen I, III Platelet derived growth factor (PDGF) : Drives HSC proliferation and migration Rapid induction both PDGE, PDGERb Hepatic injury Depletion PDGERb in HSC Decrease injury and fibrosis in CCl 4 fibrosis, BDL mouse model Vascular endothelial growth factor(VEGF) : Induce both proliferation HSC and angiogenesis Connective tissue growth factor(CTGF) : Fibrogenic cytokine Express low level in normal liver, high level in fibrotic liver mi. R-214 associate with CTGF up-regulation TWIST 1 drives mi. R-214 expression in primary mouse/human HSC cell line HSC-ECM interaction § § Integrins : transmembrane receptor; control the release and activation of TGFb - integrin av deletion protect from fibrosis ** DRUG (CWHM-12) : block of av containing integrins - integrin b 1 : regulates the profibrogenic phenotype of activated HSC - PAK 1, YAP 1 mediator of profibrotic integrinb 1 signaling DDRs (Discoidin domain-containing receptors) : Interact with collagen DDR 2 in acute hepatic injury increase fibrosis Hedgehog : Hh pathway important regulator of progenitor cell fate, injury, repair and fibrosis ∴ inhibit Hh pathway reduce fibrosis, HSC activation (1) SMO : Deletion of in cells attenuated a-SMA, ACTA 2 during liver injury (2) PTC : PTC hetero mouse+fed MCD diet increase Hh pathway, express more osteopontin develop more liver fibrosis (3) OPN (Osteropontin) : profibrogenic extracellular matrix protein - OPN deficient mouse reduce fibrosis in MCD diet, CCl 4, TAA mouse model (4) Caspase 2 - caspase 2 deficient mouse reduce pro-fibrogenic, Hh target genes Blocking Hh signaling in activated HSCs NOT ONLY inhibits liver fibrosis but also prevents accumulation of liver progenitor cells. Hh and Notch signaling might regulate fate decisions in HSC Lacking SMO in cells expressing a-SMA have blockade of Notch signaling and reduce hepatic accumulation of myofibroblasts Nat Rev Gastroenterol Hepatol. 2017 Jul; 14(7): 397 -411

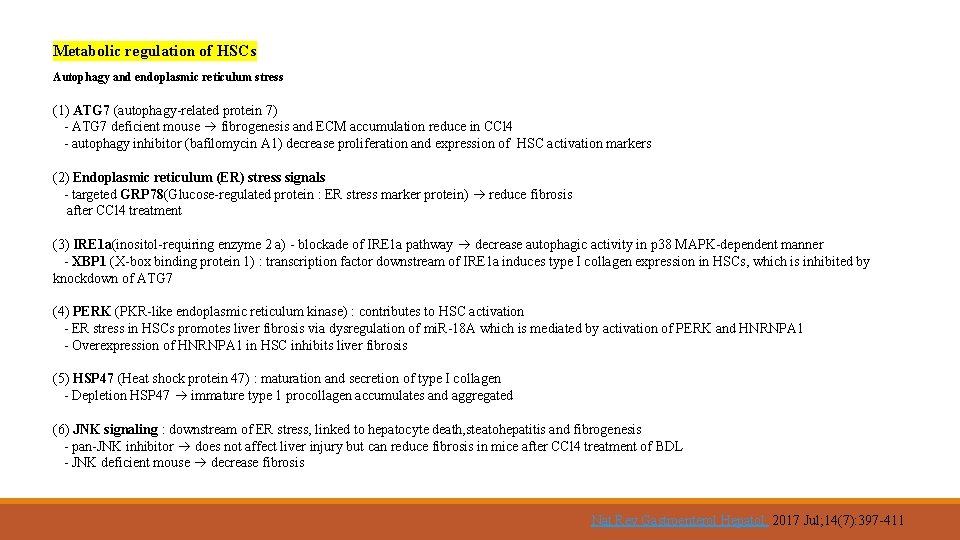
Metabolic regulation of HSCs Autophagy and endoplasmic reticulum stress (1) ATG 7 (autophagy-related protein 7) - ATG 7 deficient mouse fibrogenesis and ECM accumulation reduce in CCl 4 - autophagy inhibitor (bafilomycin A 1) decrease proliferation and expression of HSC activation markers (2) Endoplasmic reticulum (ER) stress signals - targeted GRP 78(Glucose-regulated protein : ER stress marker protein) reduce fibrosis after CCl 4 treatment (3) IRE 1 a(inositol-requiring enzyme 2 a) - blockade of IRE 1 a pathway decrease autophagic activity in p 38 MAPK-dependent manner - XBP 1 (X-box binding protein 1) : transcription factor downstream of IRE 1 a induces type I collagen expression in HSCs, which is inhibited by knockdown of ATG 7 (4) PERK (PKR-like endoplasmic reticulum kinase) : contributes to HSC activation - ER stress in HSCs promotes liver fibrosis via dysregulation of mi. R-18 A which is mediated by activation of PERK and HNRNPA 1 - Overexpression of HNRNPA 1 in HSC inhibits liver fibrosis (5) HSP 47 (Heat shock protein 47) : maturation and secretion of type I collagen - Depletion HSP 47 immature type 1 procollagen accumulates and aggregated (6) JNK signaling : downstream of ER stress, linked to hepatocyte death, steatohepatitis and fibrogenesis - pan-JNK inhibitor does not affect liver injury but can reduce fibrosis in mice after CCl 4 treatment of BDL - JNK deficient mouse decrease fibrosis Nat Rev Gastroenterol Hepatol. 2017 Jul; 14(7): 397 -411

Cellular signaling pathways involved in HSC activation and deactivation Advanced Drug Delivery Reviews 121 (2017) 27– 4

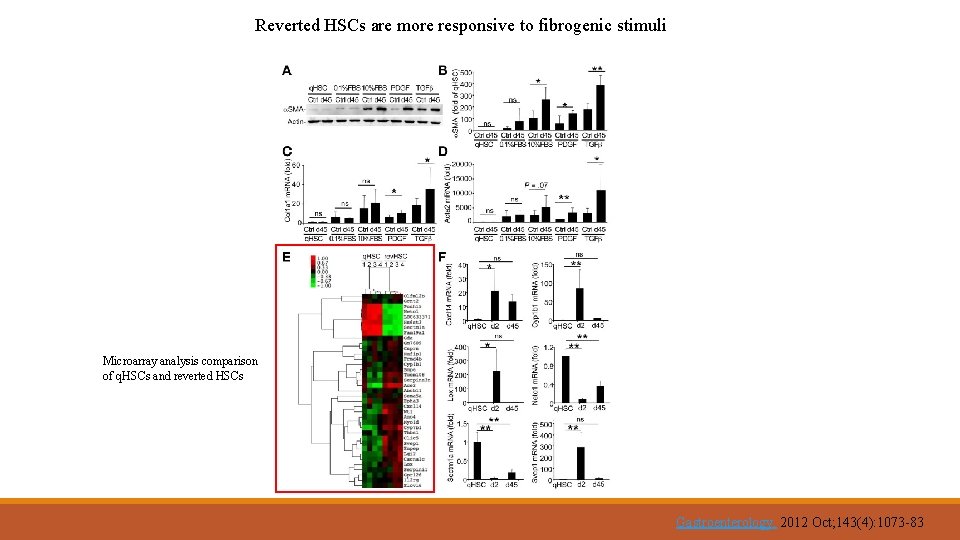
Reverted HSCs are more responsive to fibrogenic stimuli Microarray analysis comparison of q. HSCs and reverted HSCs Gastroenterology. 2012 Oct; 143(4): 1073 -83

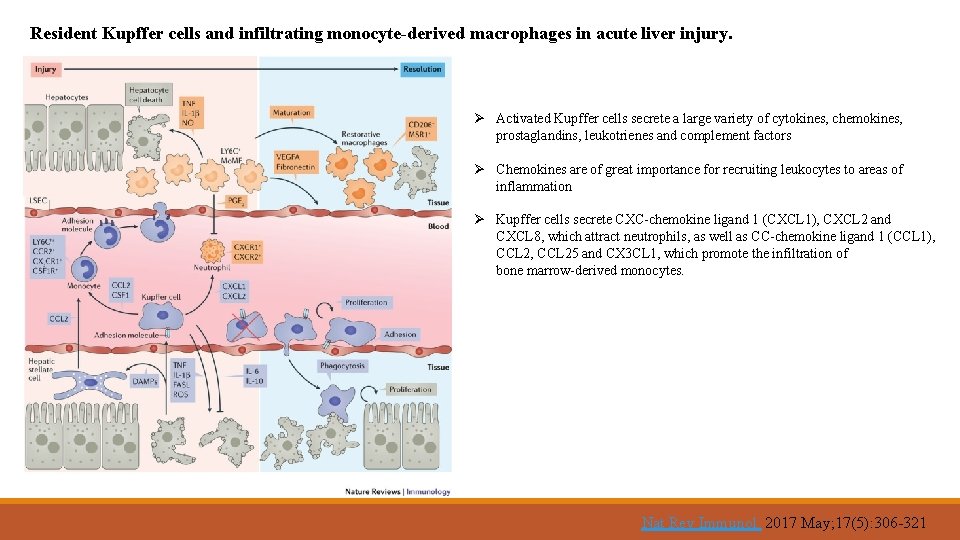
Resident Kupffer cells and infiltrating monocyte-derived macrophages in acute liver injury. Ø Activated Kupffer cells secrete a large variety of cytokines, chemokines, prostaglandins, leukotrienes and complement factors Ø Chemokines are of great importance for recruiting leukocytes to areas of inflammation Ø Kupffer cells secrete CXC-chemokine ligand 1 (CXCL 1), CXCL 2 and CXCL 8, which attract neutrophils, as well as CC-chemokine ligand 1 (CCL 1), CCL 25 and CX 3 CL 1, which promote the infiltration of bone marrow-derived monocytes. Nat Rev Immunol. 2017 May; 17(5): 306 -321

Ø CXCL 1 expression was markedly upregulated in HCC tissues Ø Ectopic expression of CXCL 1 significantly promoted HCC cells proliferation and invasion. Ø CXCL 1 promote cell invasion through NF-k. B-dependent pathway. Expression of CXCL 1 in HCC tissue samples and HCC cell lines. Representative immunohistochemical staining of CXCL 1 in HCC tissue

Ø Immune-suppressive tumour microenvironment associated with intact RIP 1/RIP 3 signalling depended in part on necroptosis-induced expression of the chemokine attractant CXCL 1, and CXCL 1 blockade protected against PDA Ø Necroptosis-induced CXCL 1 and Mincle signalling that promote macrophage-induced adaptive immune suppression and thereby enable PDA progression.

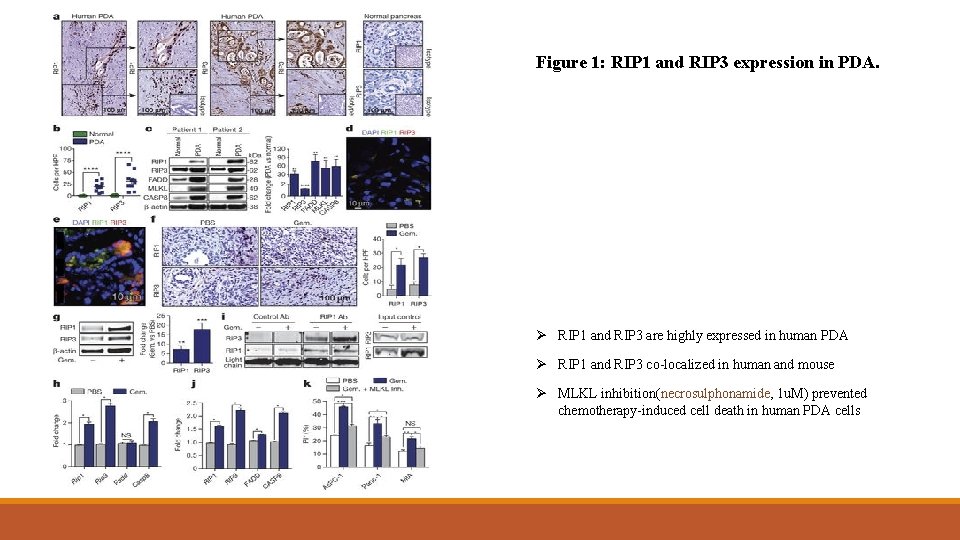
Figure 1: RIP 1 and RIP 3 expression in PDA. Ø RIP 1 and RIP 3 are highly expressed in human PDA Ø RIP 1 and RIP 3 co-localized in human and mouse Ø MLKL inhibition(necrosulphonamide, 1 u. M) prevented chemotherapy-induced cell death in human PDA cells

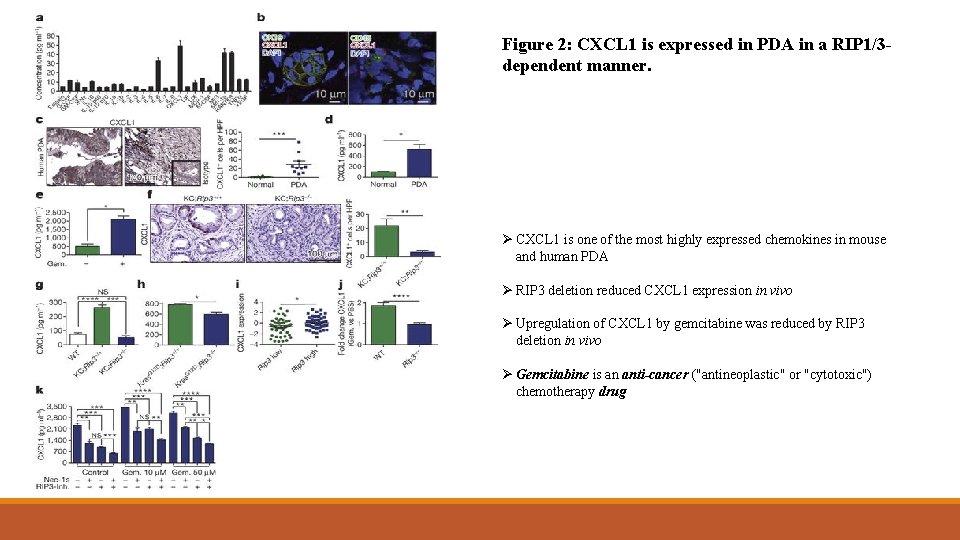
Figure 2: CXCL 1 is expressed in PDA in a RIP 1/3 dependent manner. Ø CXCL 1 is one of the most highly expressed chemokines in mouse and human PDA Ø RIP 3 deletion reduced CXCL 1 expression in vivo Ø Upregulation of CXCL 1 by gemcitabine was reduced by RIP 3 deletion in vivo Ø Gemcitabine is an anti-cancer ("antineoplastic" or "cytotoxic") chemotherapy drug

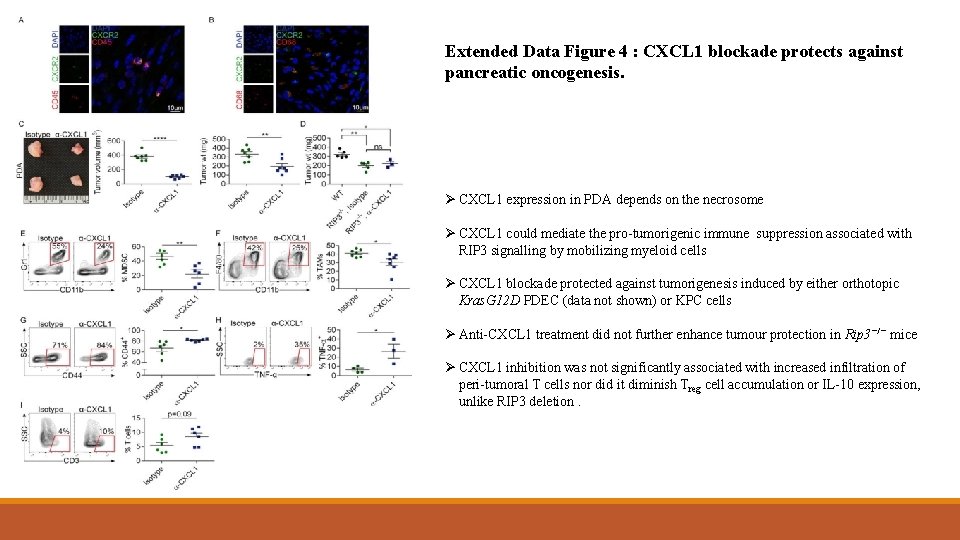
Extended Data Figure 4 : CXCL 1 blockade protects against pancreatic oncogenesis. Ø CXCL 1 expression in PDA depends on the necrosome Ø CXCL 1 could mediate the pro-tumorigenic immune suppression associated with RIP 3 signalling by mobilizing myeloid cells Ø CXCL 1 blockade protected against tumorigenesis induced by either orthotopic Kras. G 12 D PDEC (data not shown) or KPC cells Ø Anti-CXCL 1 treatment did not further enhance tumour protection in Rip 3−/− mice Ø CXCL 1 inhibition was not significantly associated with increased infiltration of peri-tumoral T cells nor did it diminish Treg cell accumulation or IL-10 expression, unlike RIP 3 deletion.
 Neural connections of cerebellum
Neural connections of cerebellum Stinging trichomes
Stinging trichomes Stellate vein
Stellate vein Hepatopancreatic ampulla.
Hepatopancreatic ampulla. Liver cells organelles
Liver cells organelles Primary target market and secondary target market
Primary target market and secondary target market Prolactin target cells
Prolactin target cells Secretin target organ
Secretin target organ Picture of target cells, dacryocytes, echinocytes.
Picture of target cells, dacryocytes, echinocytes. Waters view with open mouth
Waters view with open mouth Principal cells vs intercalated cells
Principal cells vs intercalated cells Thyroid gland
Thyroid gland How are somatic cells different from gametes
How are somatic cells different from gametes