Hemostasis Rafi Ahmed MD Hematology Oncology Fellow Hemostasis





















- Slides: 51

Hemostasis Rafi Ahmed, MD Hematology Oncology Fellow

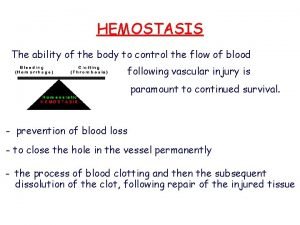
Hemostasis • Process of blood clot formation at site of vessel injury • Response must be ▫ Quick ▫ Localized ▫ Carefully regulated

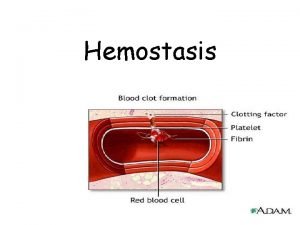
Phases of hemostasis • Formation of platelet plug • Propagation of clotting process by coagulation cascade • Termination of clotting by antithombotic control mechanisms • Removal of clot by fibrinolysis

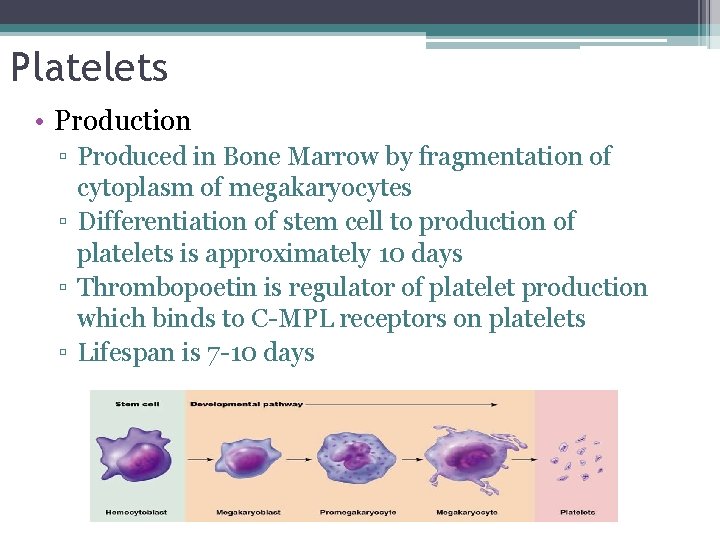
Platelets • Production ▫ Produced in Bone Marrow by fragmentation of cytoplasm of megakaryocytes ▫ Differentiation of stem cell to production of platelets is approximately 10 days ▫ Thrombopoetin is regulator of platelet production which binds to C-MPL receptors on platelets ▫ Lifespan is 7 -10 days

Platelet Structure • Glycoproteins of the surface involved in platelet reactions of adhesion and aggregation • Interior with calcium, ADP, ATP, serotonin in granules. • Alpha granules with heparin antagonist, PDGF, Beta -thromboglobulin, fibrinogen, VWF, and clotting factors

Platelet Function • Formation of mechanical plugs during vascular injury ▫ ▫ Adhesion Aggregation Secretion Procoagulant activity


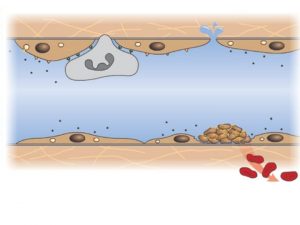
The Coagulation Cascade Primary Hemostasis • Consists of: ▫ Platelet plug formations ▫ Vascular spasm ▫ Capillary endothelial adhesions • Temporary Fix ▫ Lasts 12 -24 hours

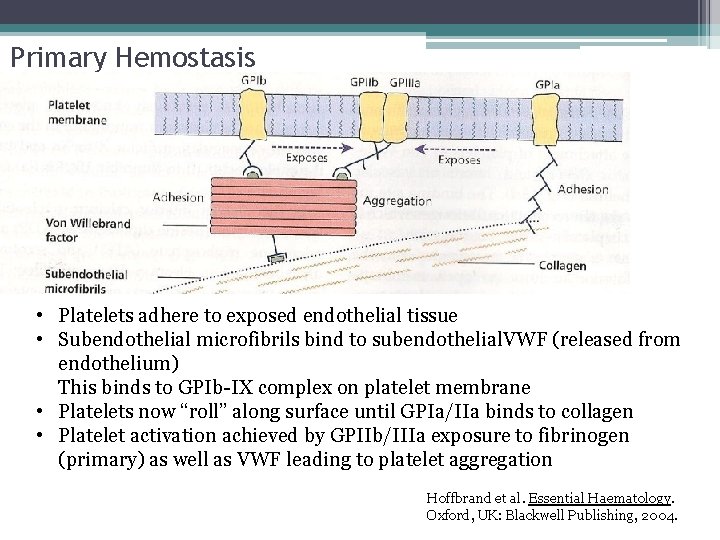
Primary Hemostasis • Platelets adhere to exposed endothelial tissue • Subendothelial microfibrils bind to subendothelial VWF (released from endothelium) This binds to GPIb-IX complex on platelet membrane • Platelets now “roll” along surface until GPIa/IIa binds to collagen • Platelet activation achieved by GPIIb/IIIa exposure to fibrinogen (primary) as well as VWF leading to platelet aggregation Hoffbrand et al. Essential Haematology. Oxford, UK: Blackwell Publishing, 2004.


Collagen Receptor GP IB/IIA GP IIB/IIIA


Primary Hemostasis • Platelets adhere to exposed endothelial tissue • Subendothelial microfibrils bind to subendothelial. VWF (released from endothelium) This binds to GPIb-IX complex on platelet membrane • Platelets now “roll” along surface until GPIa/IIa binds to collagen • Platelet activation achieved by GPIIb/IIIa exposure to fibrinogen (primary) as well as VWF leading to platelet aggregation Hoffbrand et al. Essential Haematology. Oxford, UK: Blackwell Publishing, 2004.

Primary Hemostasis: Platelet release reaction and aggregation • Collagen exposure/thrombin action: ▫ Secretion of platelet granules �ADP, serotonin, fibrinogen, lysosomal enzymes, B thromboglobulin, and heparin neutralizing factors • Activation of platelet prostaglandin synthesis ▫ Arachidonate release leads to thromboxane A 2 • Thromboxane A 2 ▫ Potentiates platelet aggregation ▫ Vasoconstrictive activity • Procoagulant Activity ▫ Phospholipid mediated reactions ▫ Factors IXa, VIIIa, and X in the formation of factor Xa ▫ Factors Xa, Va, and prothrombin II results in thrombin Hoffbrand et al. Essential Haematology. Oxford, UK: Blackwell Publishing, 2004.



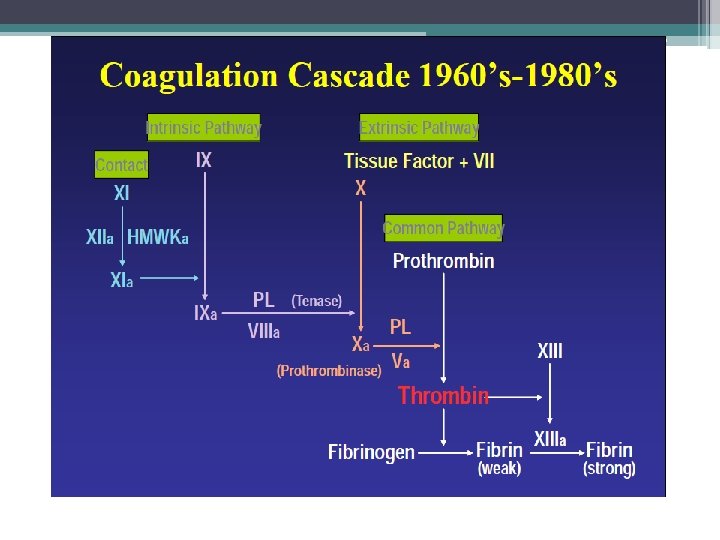
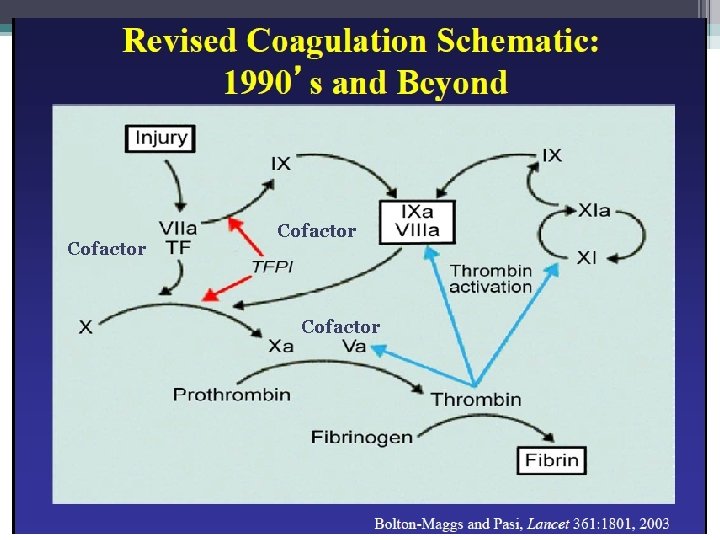
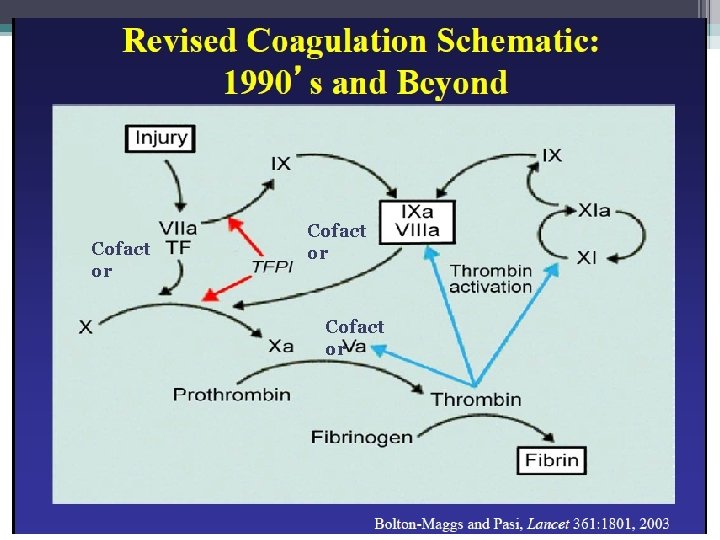
The Coagulation Cascade Secondary Hemostasis • Same time as platelets are aggregating • Initiation substances sequentially activate by proteolysis a cascade of circulating precursor proteins • Factors XII, XI, IX, VIII form the intrinsic pathway • VIIa-Tissue Factor complex form the extrinsic pathway • Both pathways leads to factor X activation • Final pathway is converting prothrombin to thrombin which converts fibrinogen to fibrin • Leads to firm and stable plugs

Secondary Hemostasis PTT PT Image retrieved from: http: //en. wikipedia. org/wiki/Coagulation



Cofactor

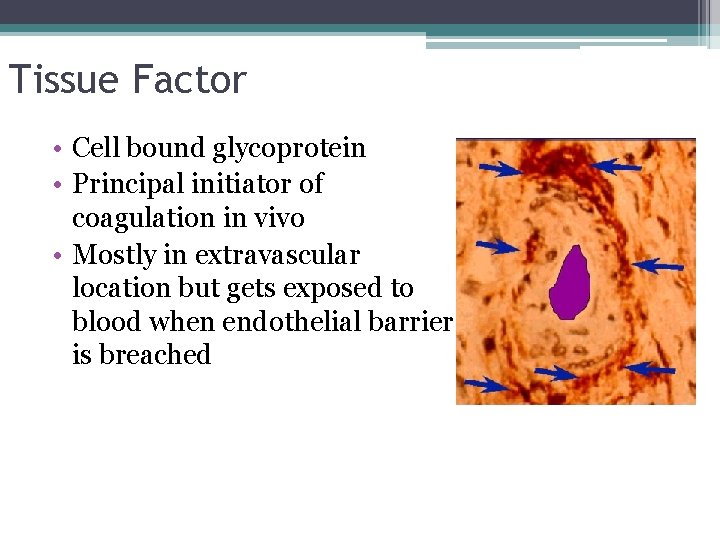
Tissue Factor • Cell bound glycoprotein • Principal initiator of coagulation in vivo • Mostly in extravascular location but gets exposed to blood when endothelial barrier is breached

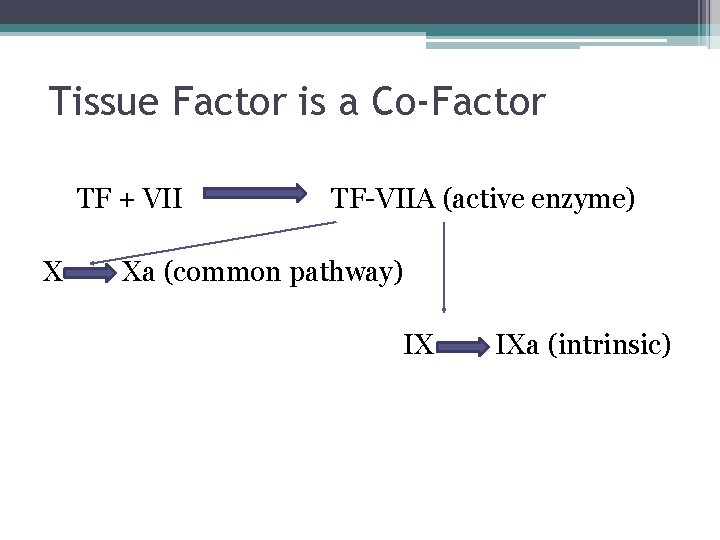
Tissue Factor is a Co-Factor TF + VII X TF-VIIA (active enzyme) Xa (common pathway) IX IXa (intrinsic)

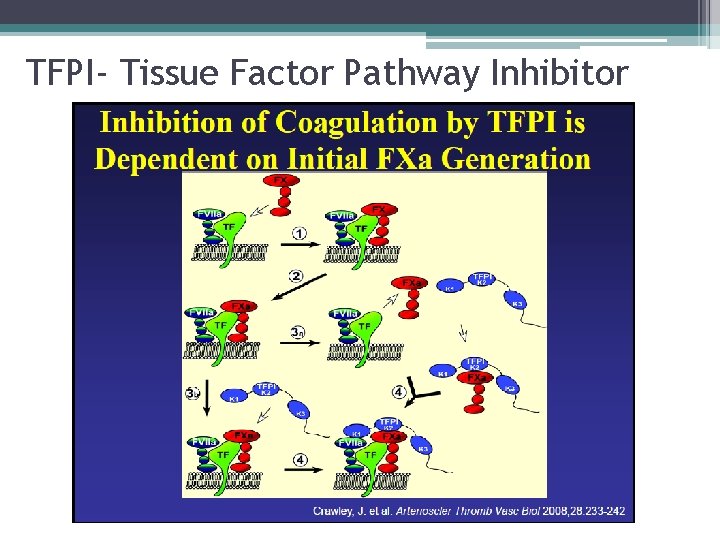
TFPI- Tissue Factor Pathway Inhibitor

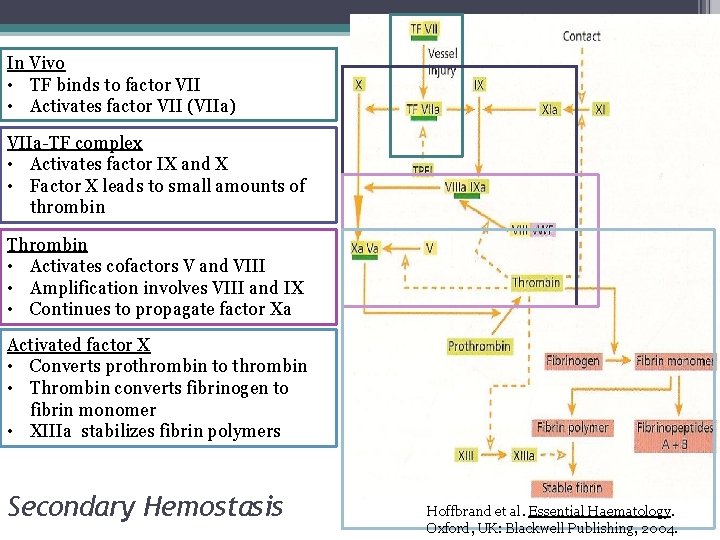
In Vivo • TF binds to factor VII • Activates factor VII (VIIa) VIIa-TF complex • Activates factor IX and X • Factor X leads to small amounts of thrombin Thrombin • Activates cofactors V and VIII • Amplification involves VIII and IX • Continues to propagate factor Xa Activated factor X • Converts prothrombin to thrombin • Thrombin converts fibrinogen to fibrin monomer • XIIIa stabilizes fibrin polymers Secondary Hemostasis Hoffbrand et al. Essential Haematology. Oxford, UK: Blackwell Publishing, 2004.

Cofact or

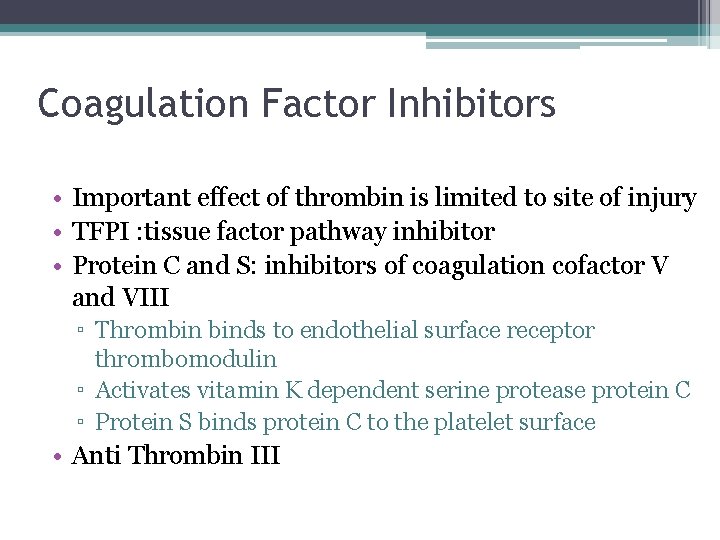
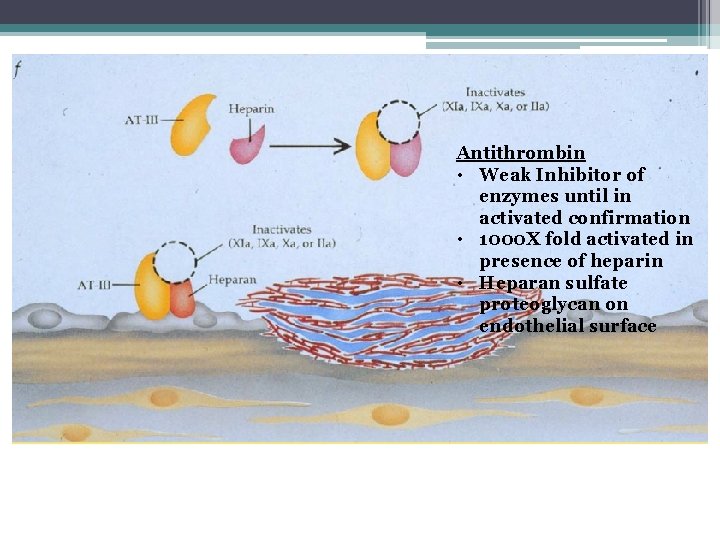
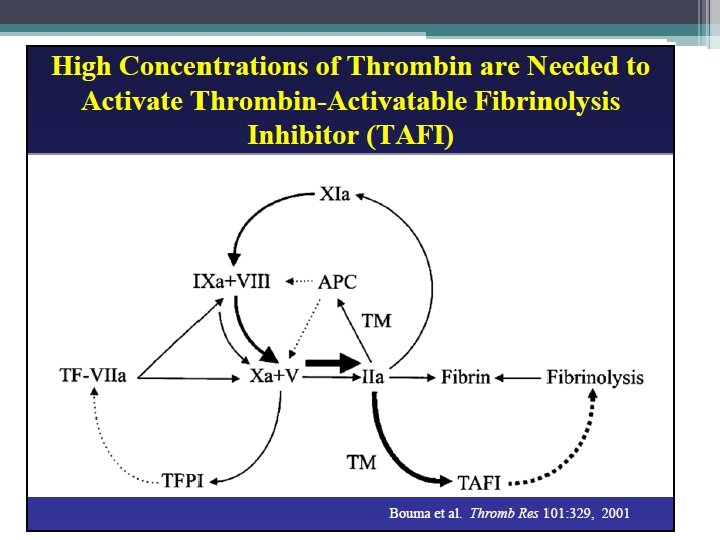
Coagulation Factor Inhibitors • Important effect of thrombin is limited to site of injury • TFPI : tissue factor pathway inhibitor • Protein C and S: inhibitors of coagulation cofactor V and VIII ▫ Thrombin binds to endothelial surface receptor thrombomodulin ▫ Activates vitamin K dependent serine protease protein C ▫ Protein S binds protein C to the platelet surface • Anti Thrombin III

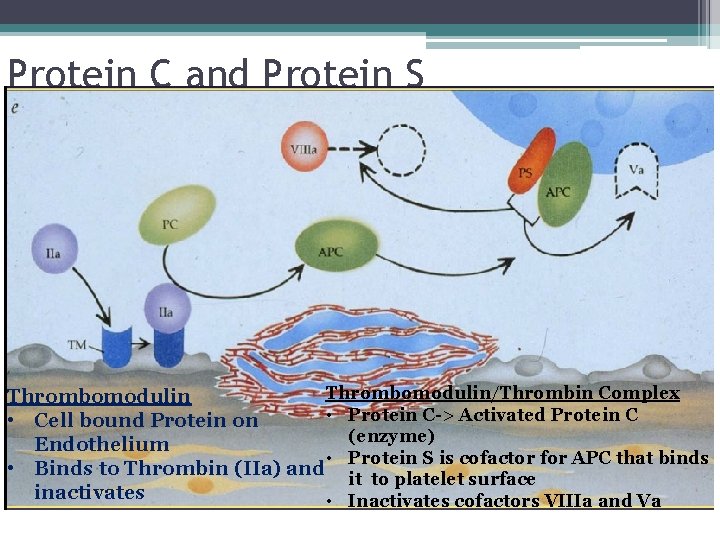
Protein C and Protein S Thrombomodulin/Thrombin Complex Thrombomodulin • Protein C-> Activated Protein C • Cell bound Protein on (enzyme) Endothelium • Protein S is cofactor for APC that binds • Binds to Thrombin (IIa) and it to platelet surface inactivates • Inactivates cofactors VIIIa and Va

Antithrombin III Antithrombin • Weak Inhibitor of enzymes until in activated confirmation • 1000 X fold activated in presence of heparin • Heparan sulfate proteoglycan on endothelial surface

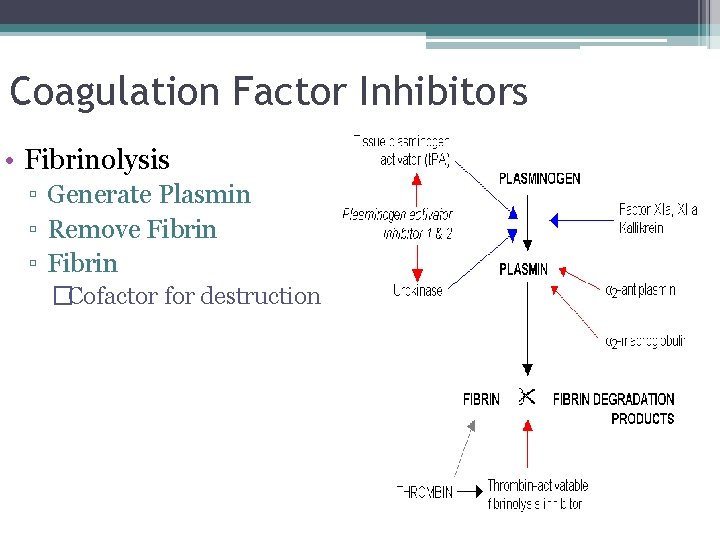
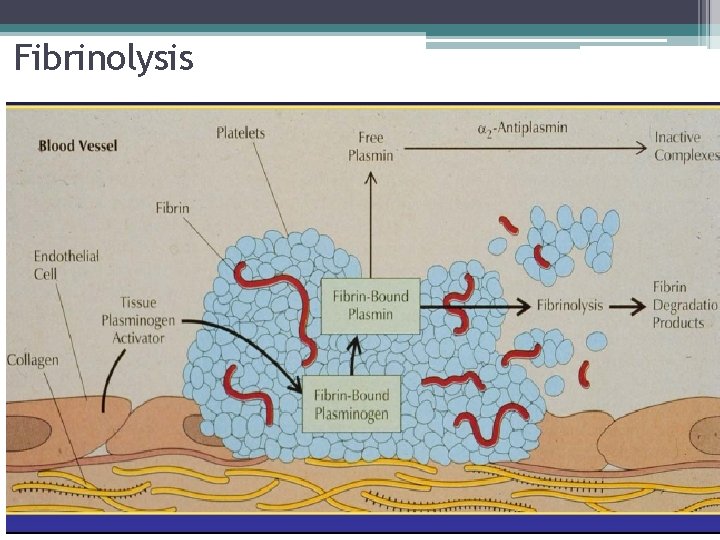
Coagulation Factor Inhibitors • Fibrinolysis ▫ Generate Plasmin ▫ Remove Fibrin ▫ Fibrin �Cofactor for destruction


Fibrinolysis

Approach to Bleeding Patient • Abnormal Bleeding May Result From ▫ ▫ Vascular disorders Thrombocytopenia Defective platelet function Defective coagulation

Pattern of Bleeding • Vascular and Platelet Disorders ▫ Associated with bleeding from mucous membranes and into skin • Coagulation Disorders ▫ Bleeding in soft tissue or joints

Patient History • Bleeding History ▫ Past bleeding ▫ During surgical procedures ▫ Mucosal vs hemarthrosis • Medication Use ▫ Aspirin, clopidogrel, ticlopidine, warfarin

Inherited Vascular Disorders • Hereditary hemorrhagic telangiectasia ▫ ▫ Autosomal dominant Dilated microvascular swellings Pulmonary AV malformations Recurrent GI hemorrhage • Connective Tissue Disorders ▫ Ehlers-Danlos �Hereditary collagen abnormalities �Defective platelet aggregation

Acquired Vascular Defects • Senile purpura ▫ Atrophy of supporting tissues of cutaneous blood vessels on dorsal aspects of forearms and hands • Purpura due to infections ▫ Vascular damage from immune complex formation • Henoch-Schonlein ▫ In children, follows acute infection ▫ Ig. A mediated vasculitis • Scurvy ▫ Vitamin C deficiency, defective collagen • Steroid purpura ▫ Defective vascular support

Thrombocytopenia • Production Defects ▫ Bone Marrow Failure • Increased Destruction ▫ ▫ ▫ ITP HIT Drug induced TTP/HUS DIC Splenic Pooling

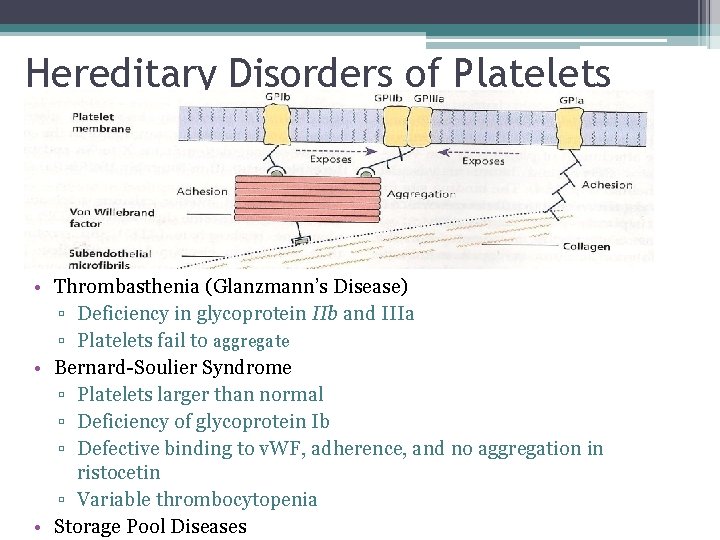
Hereditary Disorders of Platelets • Thrombasthenia (Glanzmann’s Disease) ▫ Deficiency in glycoprotein IIb and IIIa ▫ Platelets fail to aggregate • Bernard-Soulier Syndrome ▫ Platelets larger than normal ▫ Deficiency of glycoprotein Ib ▫ Defective binding to v. WF, adherence, and no aggregation in ristocetin ▫ Variable thrombocytopenia • Storage Pool Diseases

Acquired Platelet Functional Disorders • Liver disease ▫ Decrease in clotting factors ▫ Quantitative and qualitative platelet disorders ▫ Cirrhosis: sequestration, decreased TPO • Uremia ▫ Ecchymosis, epistaxis, GI/GU bleeding • Cardiopulmonary Bypass ▫ Platelet interaction with non-physiologic surfaces ▫ Hypothermia, complement activation, cytokines, thrombin ▫ Adhesive properties altered

Von Willebrands Disease • VWF Functions ▫ Primary Hemostasis �Platelet Adhesion �Platelet Aggregation ▫ Secondary Hemostasis �Carrier of VIII in plasma (keeps it inactivated) until thrombin releases it ▫ Synthesized in endothelial cells and megakaryocytes • Von Willebrand Disease ▫ Autosomal dominant

Von Willebrand Disease • Mucocutaneous bleeding usually mild to moderate • Assays ▫ VWF activity: measures VWF binding to plts �Ristocetin cofactor assay-gold standard �Collagen binding, VWF: activity assay ▫ VWF antigen: concentration of protein (elisa) ▫ Factor VIII activity (carrier function of VWF) ▫ VWF Multimers, RIPA (Assays for classification) • Type 1: Partial quantitative deficiency of VWF • Type 2: (A, B, M, N) Qualitative defect in VWF • Type 3: Total deficiency of VWF

Quick Review of Hemostasis Screening Assays • • Thrombin Time PT PTT Mixing Study

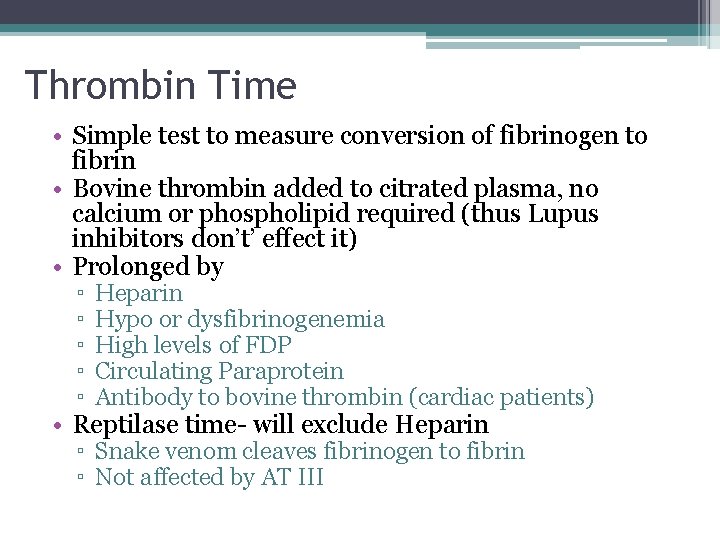
Thrombin Time • Simple test to measure conversion of fibrinogen to fibrin • Bovine thrombin added to citrated plasma, no calcium or phospholipid required (thus Lupus inhibitors don’t’ effect it) • Prolonged by ▫ ▫ ▫ Heparin Hypo or dysfibrinogenemia High levels of FDP Circulating Paraprotein Antibody to bovine thrombin (cardiac patients) • Reptilase time- will exclude Heparin ▫ Snake venom cleaves fibrinogen to fibrin ▫ Not affected by AT III

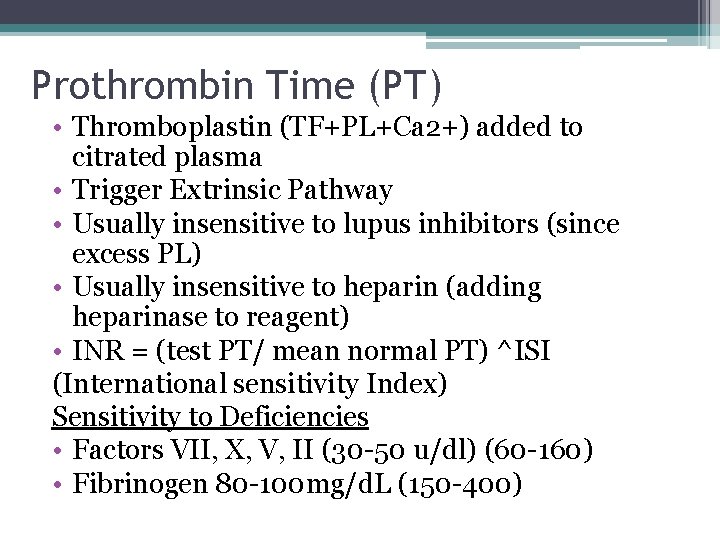
Prothrombin Time (PT) • Thromboplastin (TF+PL+Ca 2+) added to citrated plasma • Trigger Extrinsic Pathway • Usually insensitive to lupus inhibitors (since excess PL) • Usually insensitive to heparin (adding heparinase to reagent) • INR = (test PT/ mean normal PT) ^ISI (International sensitivity Index) Sensitivity to Deficiencies • Factors VII, X, V, II (30 -50 u/dl) (60 -160) • Fibrinogen 80 -100 mg/d. L (150 -400)

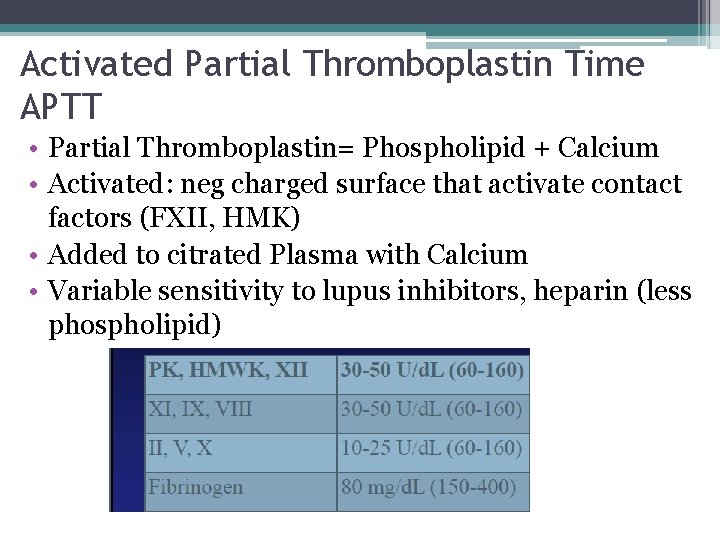
Activated Partial Thromboplastin Time APTT • Partial Thromboplastin= Phospholipid + Calcium • Activated: neg charged surface that activate contact factors (FXII, HMK) • Added to citrated Plasma with Calcium • Variable sensitivity to lupus inhibitors, heparin (less phospholipid)

Mixing Study • 50 -50 mix with normal plasma will correct if due to factor deficiency (50% of nl levels of any component factor) • If not correct then concern for inhibitor • Sometimes corrects but after incubation becomes prolonged- concern for inhibitor

Nl PT and prolonged PTT Disorders of intrinsic pathway Deficiencies • Factor VIII: Hemophilia A, VWD • Factor IX: Hemophilia B • Factor XI (variable bleeding history) • Factor XII, prekallikrein, HMWK (not associated with bleeding) Inhibitors • Factor VIII (most common) • Factor IX, XI (rare)

Prolonged PT and normal PTT • Deficiency ▫ Factor VII deficiency (range of phenotype) • Acquired inhibitors ▫ Factor VII rare • Most commonly seen in warfarin therapy, early liver disease, vitamin K deficiency, DIC

Prolonged PT and PTT • Deficiency of Factor X, V, II, I • Super therapeutic warfarin or heparin • Vitamin K deficiency, liver disease, DIC, fibrinolysis

And finally some anticoagulants • Vitamin K antagonists ▫ Warfarin (factor 10, 9, 7, 2, C, S) • Activates Antithrombin III ▫ Heparin, LMWH ▫ Fondaparinux (pentasacchride) • Direct Xa Inhibitors ▫ Rivaroxaban, apixaban • Direct Thrombin Inhibitors ▫ Argatroban, Dabigatran
 Ahmed muhudiin ahmed
Ahmed muhudiin ahmed Oncological emergencies wikipedia
Oncological emergencies wikipedia Unc benign hematology
Unc benign hematology Ser medical term prefix
Ser medical term prefix Pediatric hematology oncology board review questions
Pediatric hematology oncology board review questions Umkc gme
Umkc gme Achog
Achog Irish nodülü
Irish nodülü Rafi is pulling a toy wagon
Rafi is pulling a toy wagon Investing plug
Investing plug 1ry hemostasis prescribed by
1ry hemostasis prescribed by Hemostasis
Hemostasis Hemostasis
Hemostasis Skema hemostasis
Skema hemostasis Alpha granules of platelets
Alpha granules of platelets Coagulation factors
Coagulation factors Primary hemostasis
Primary hemostasis Surgicutt bleeding time device
Surgicutt bleeding time device Hemostasis
Hemostasis Hemostasis process
Hemostasis process Ecchumosis
Ecchumosis Primary hemostasis
Primary hemostasis Saddle blood clot
Saddle blood clot Odd fellow orden
Odd fellow orden Nhmrc emerging leadership fellow
Nhmrc emerging leadership fellow Asme fellow nomination
Asme fellow nomination Paul harris fellow certificate template
Paul harris fellow certificate template Odd fellow medlemsregister
Odd fellow medlemsregister Ieee fellow nomination
Ieee fellow nomination Little fellow
Little fellow Fellow lpkia
Fellow lpkia Ieee fellow program
Ieee fellow program 2bee3
2bee3 Customer contributions and roles in service delivery
Customer contributions and roles in service delivery Fellow lpkia
Fellow lpkia Poor little black fellow
Poor little black fellow Main submitter speech
Main submitter speech Clarence filsfils
Clarence filsfils Branches of hematology
Branches of hematology Hematopoietic drugs wikipedia
Hematopoietic drugs wikipedia Hematology
Hematology Reticulocyte production index calculator
Reticulocyte production index calculator Unacceptable blood smears
Unacceptable blood smears Diagnostic suffixes examples
Diagnostic suffixes examples Surgical suffixes examples
Surgical suffixes examples Hematology day
Hematology day Hemolyser
Hemolyser Hemolyser
Hemolyser Blok diagram hematology analyzer
Blok diagram hematology analyzer Hypochromic red cell
Hypochromic red cell Retic count
Retic count Hematology laboratory procedures
Hematology laboratory procedures