HEMOGLOBIN BIOCHEMISTRY BMS 233 L NOHA SOLIMAN Objectives
HEMOGLOBIN BIOCHEMISTRY (BMS 233) L. NOHA SOLIMAN

Objectives Ø After studying this chapter, you should be able to: • Describe the most important structural similarities and differences between myoglobin and hemoglobin. • Sketch binding curves for the oxygenation of myoglobin and hemoglobin. • Identify the covalent linkages and other close associations between heme and globin in oxymyoglobin and oxyhemoglobin. • Explain why the physiologic function of hemoglobin requires that its O 2 binding curve be sigmoidal rather than hyperbolic. • Define P 50 and indicate its significance in oxygen transport and delivery. • Describe the structural and conformational changes in hemoglobin that accompany its oxygenation and subsequent deoxygenation. • Explain the role of 2, 3 -bisphoglycerate (BPG) in oxygen binding and delivery. • Outline the role of hemoglobin in CO 2 and proton transport.
BIOMEDICAL IMPORTANCE Ø The efficient delivery of oxygen from the lungs to the peripheral tissues and the maintenance of tissue reserves to protect against anoxic episodes are essential to health. Ø In mammals, these functions are performed by the homologous heme proteins hemoglobin and myoglobin, respectively. Ø Myoglobin, a monomeric protein of red muscle, binds oxygen tightly as a reserve against oxygen deprivation. Ø The multiple subunits of hemoglobin, a tetrameric protein of erythrocytes, interact in a cooperative fashion that enables this transporter to offload a high proportion of bound O 2 in peripheral tissues while simultaneously retaining the capacity to bind it efficiently in the lungs. Ø In addition to delivering O 2, hemoglobin scavenges the waste products of respiration, CO 2 and protons, for transport to and ultimate disposal in the lungs
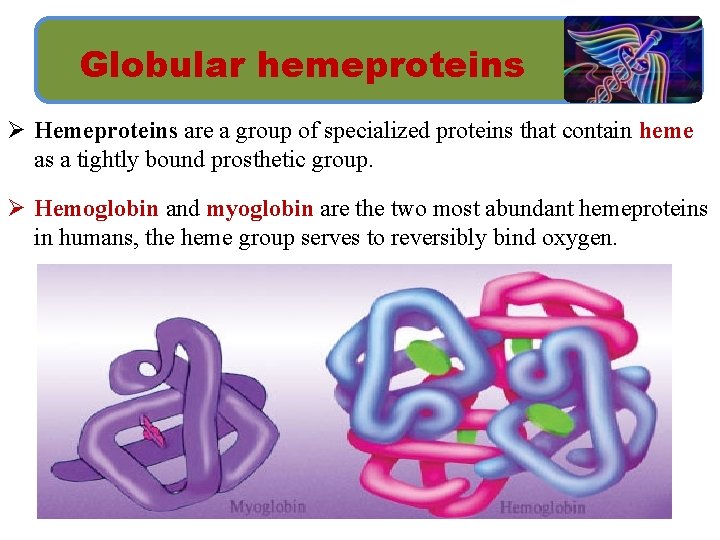
Globular hemeproteins Ø Hemeproteins are a group of specialized proteins that contain heme as a tightly bound prosthetic group. Ø Hemoglobin and myoglobin are the two most abundant hemeproteins in humans, the heme group serves to reversibly bind oxygen.
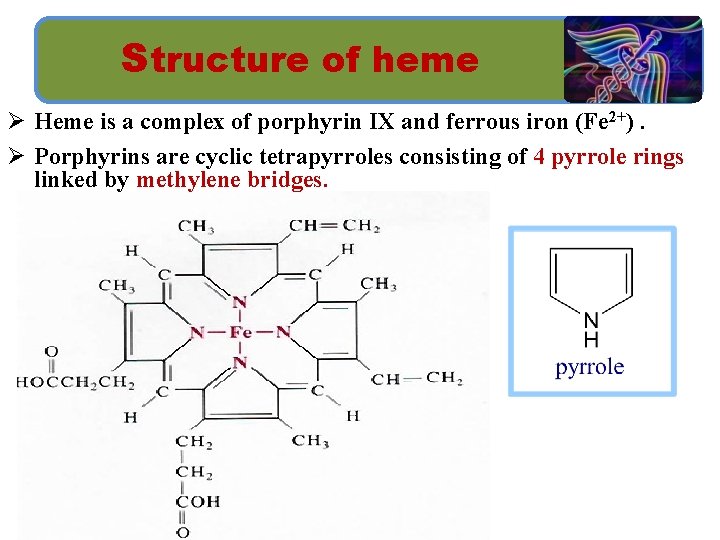
Structure of heme Ø Heme is a complex of porphyrin IX and ferrous iron (Fe 2+). Ø Porphyrins are cyclic tetrapyrroles consisting of 4 pyrrole rings linked by methylene bridges.
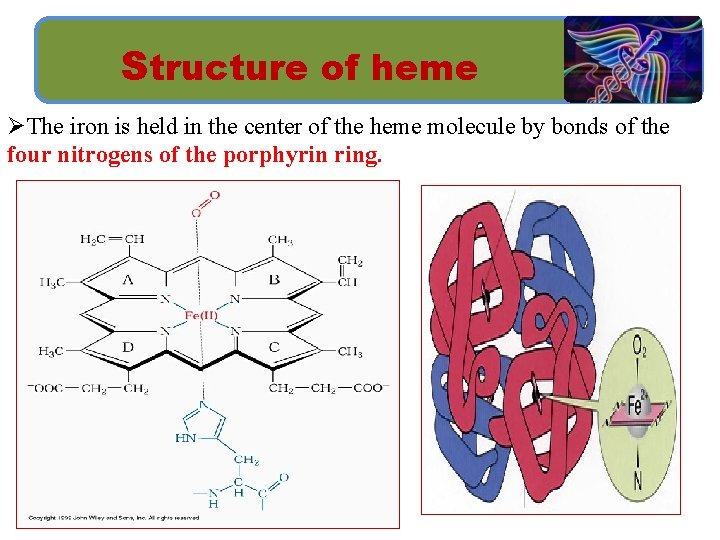
Structure of heme ØThe iron is held in the center of the heme molecule by bonds of the four nitrogens of the porphyrin ring.
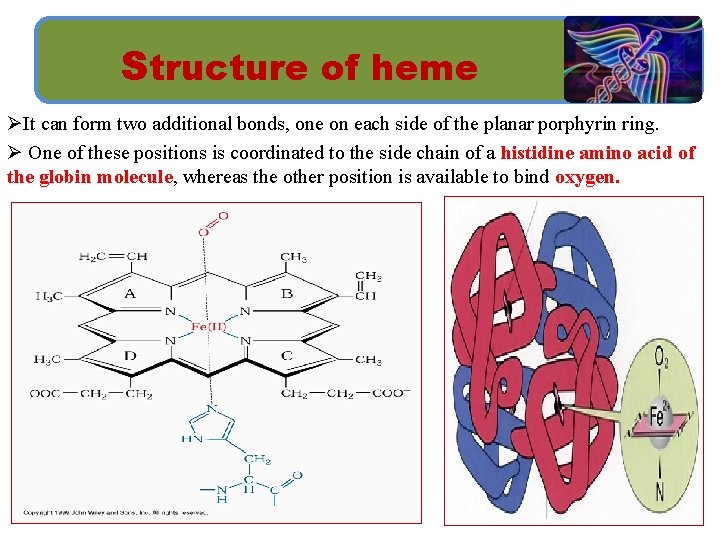
Structure of heme ØIt can form two additional bonds, one on each side of the planar porphyrin ring. Ø One of these positions is coordinated to the side chain of a histidine amino acid of the globin molecule, whereas the other position is available to bind oxygen.
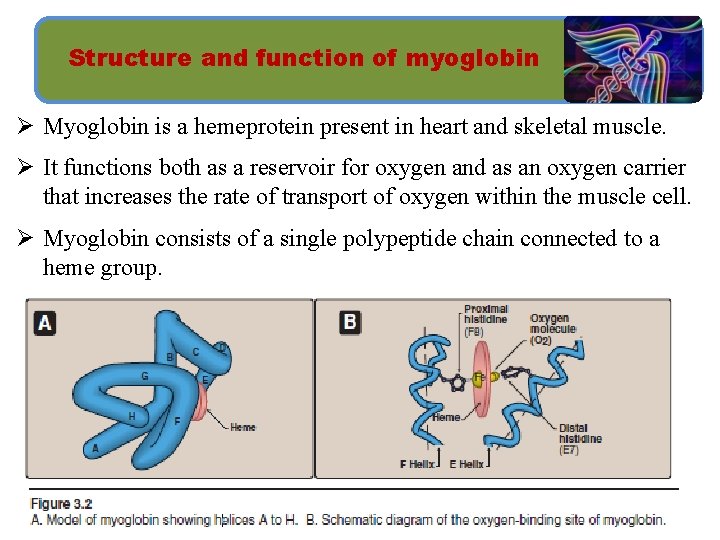
Structure and function of myoglobin Ø Myoglobin is a hemeprotein present in heart and skeletal muscle. Ø It functions both as a reservoir for oxygen and as an oxygen carrier that increases the rate of transport of oxygen within the muscle cell. Ø Myoglobin consists of a single polypeptide chain connected to a heme group.
Structure and function of hemoglobin • Hemoglobin is found exclusively in red blood cells (RBC), where its main function is to transport oxygen (O 2) from the lungs to the cells of the body. • Hemoglobin can transport H+ and CO 2 from the tissues to the lungs.

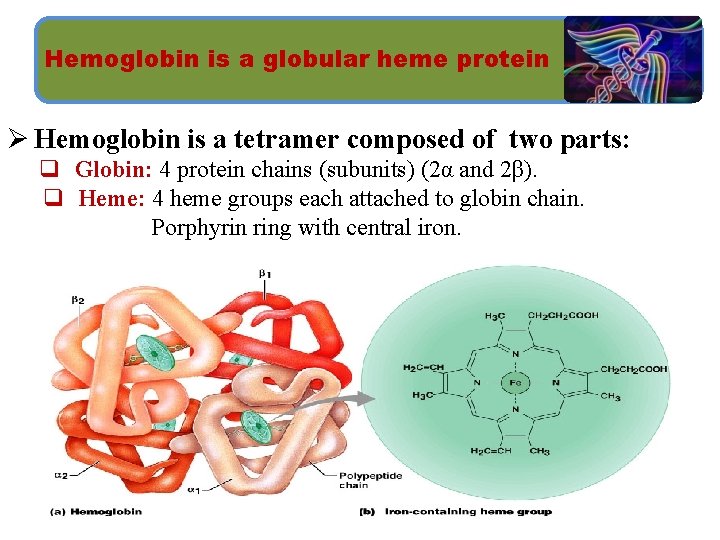
Hemoglobin is a globular heme protein Ø Hemoglobin is a tetramer composed of two parts: q Globin: 4 protein chains (subunits) (2α and 2β). q Heme: 4 heme groups each attached to globin chain. Porphyrin ring with central iron.
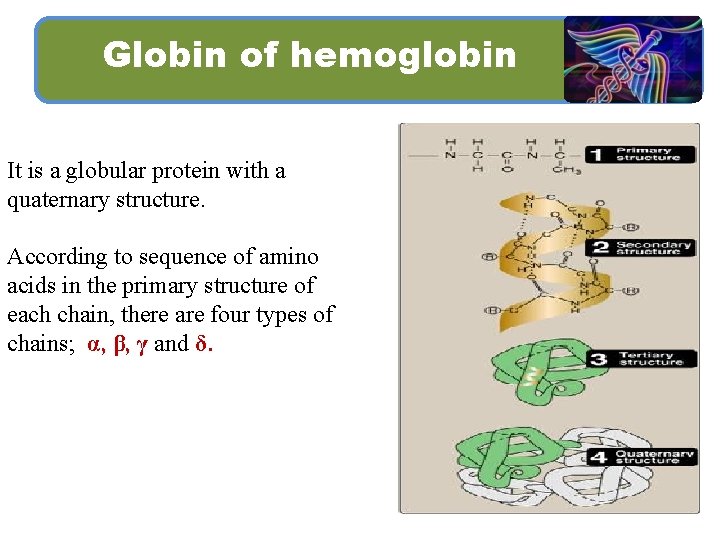
Globin of hemoglobin It is a globular protein with a quaternary structure. According to sequence of amino acids in the primary structure of each chain, there are four types of chains; α, β, γ and δ.
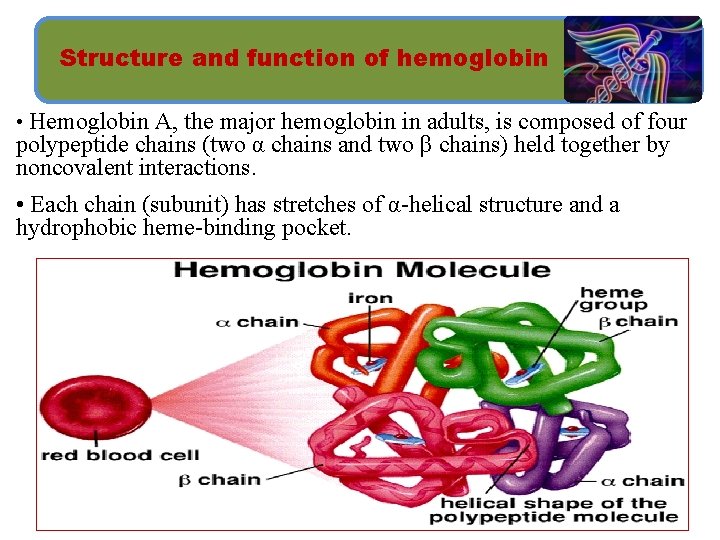
Structure and function of hemoglobin • Hemoglobin A, the major hemoglobin in adults, is composed of four polypeptide chains (two α chains and two β chains) held together by noncovalent interactions. • Each chain (subunit) has stretches of α-helical structure and a hydrophobic heme-binding pocket.
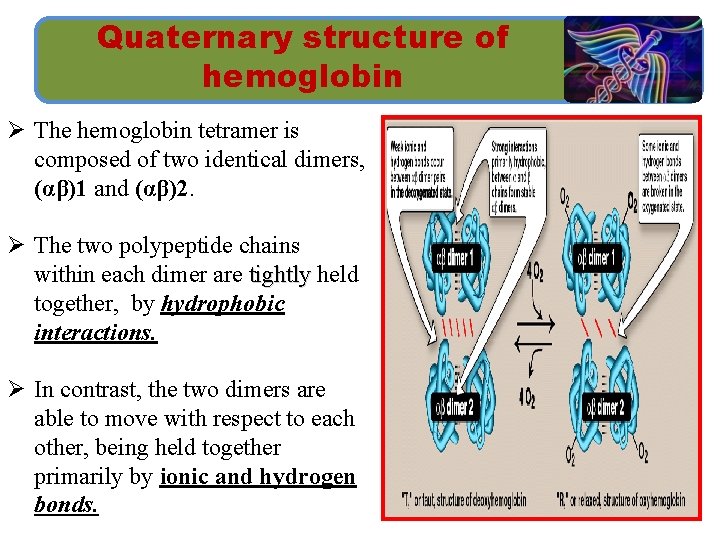
Quaternary structure of hemoglobin Ø The hemoglobin tetramer is composed of two identical dimers, (αβ)1 and (αβ)2. Ø The two polypeptide chains within each dimer are tightly held together, by hydrophobic interactions. Ø In contrast, the two dimers are able to move with respect to each other, being held together primarily by ionic and hydrogen bonds.
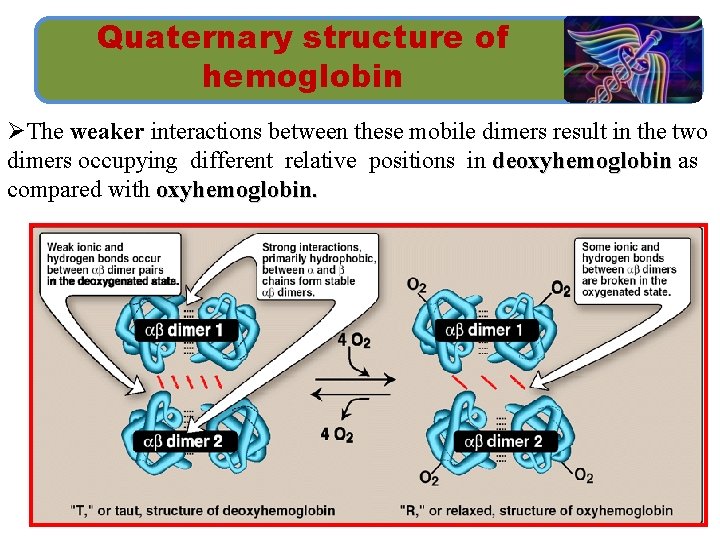
Quaternary structure of hemoglobin ØThe weaker interactions between these mobile dimers result in the two dimers occupying different relative positions in deoxyhemoglobin as compared with oxyhemoglobin.
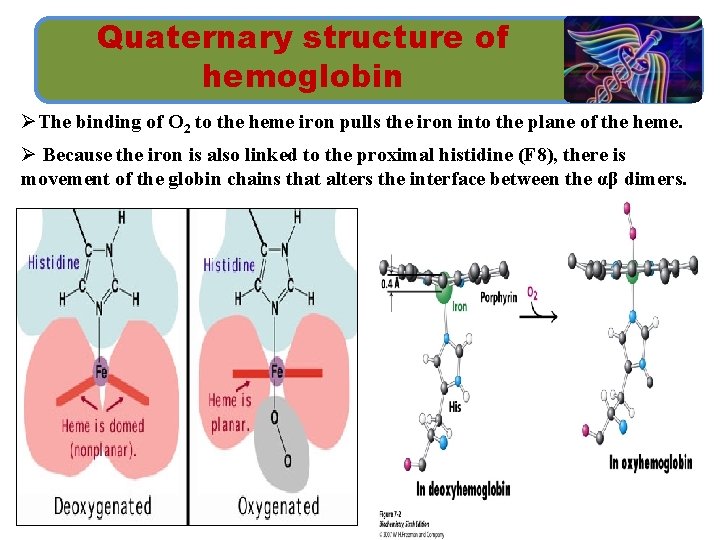
Quaternary structure of hemoglobin ØThe binding of O 2 to the heme iron pulls the iron into the plane of the heme. Ø Because the iron is also linked to the proximal histidine (F 8), there is movement of the globin chains that alters the interface between the αβ dimers.
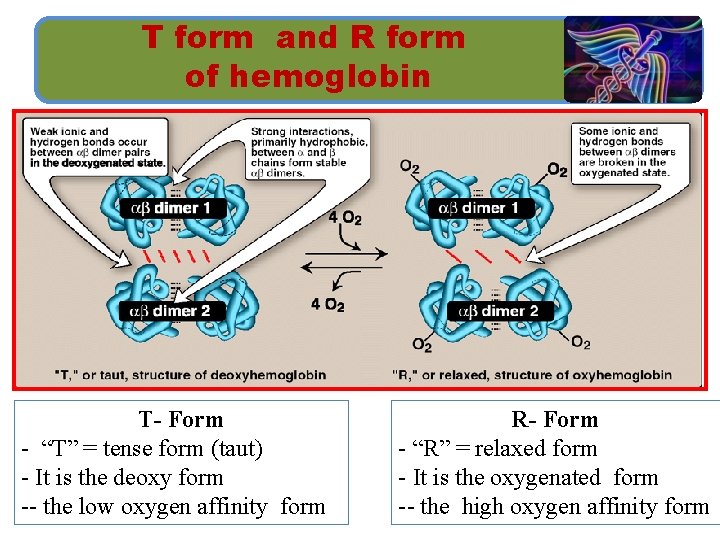
T form and R form of hemoglobin T- Form - “T” = tense form (taut) - It is the deoxy form -- the low oxygen affinity form R- Form - “R” = relaxed form - It is the oxygenated form -- the high oxygen affinity form

Binding of oxygen to myoglobin and hemoglobin Ø Myoglobin can bind only one molecule of O 2, because it contains only one heme group. Ø In contrast, hemoglobin can bind four O 2 molecules, one at each of its four heme groups. Ø The degree of saturation (Y) of these oxygen-binding sites on all myoglobin or hemoglobin molecules can vary between zero (all sites are empty) and 100% (all sites are full).
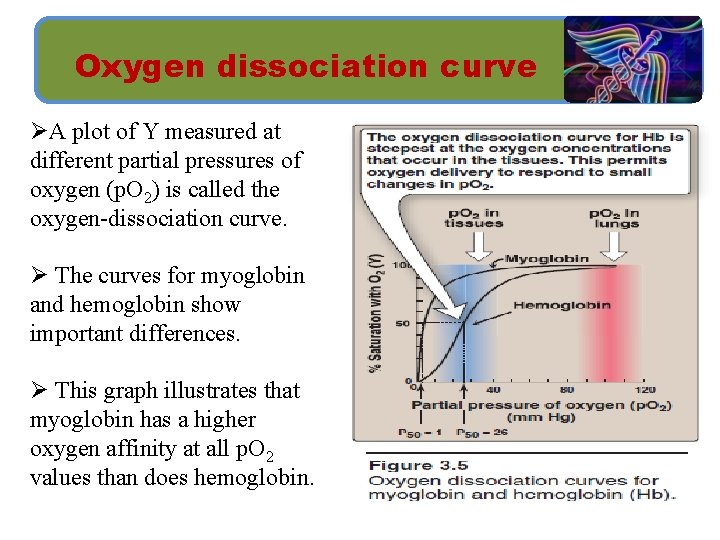
Oxygen dissociation curve ØA plot of Y measured at different partial pressures of oxygen (p. O 2) is called the oxygen-dissociation curve. Ø The curves for myoglobin and hemoglobin show important differences. Ø This graph illustrates that myoglobin has a higher oxygen affinity at all p. O 2 values than does hemoglobin.
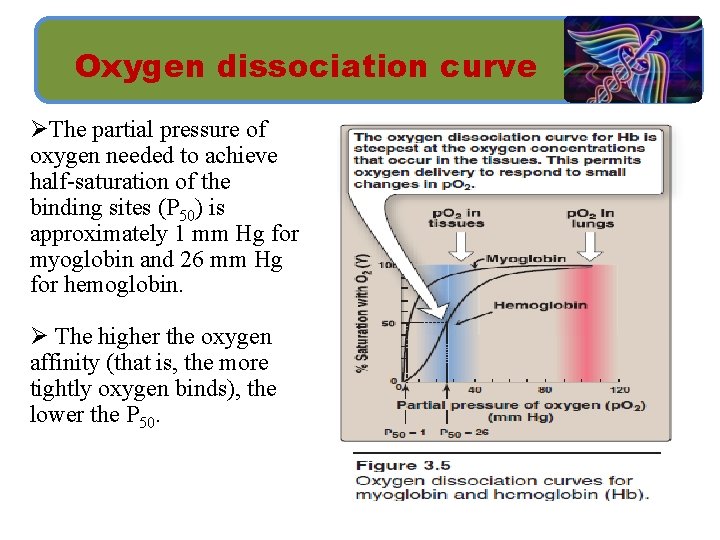
Oxygen dissociation curve ØThe partial pressure of oxygen needed to achieve half-saturation of the binding sites (P 50) is approximately 1 mm Hg for myoglobin and 26 mm Hg for hemoglobin. Ø The higher the oxygen affinity (that is, the more tightly oxygen binds), the lower the P 50.
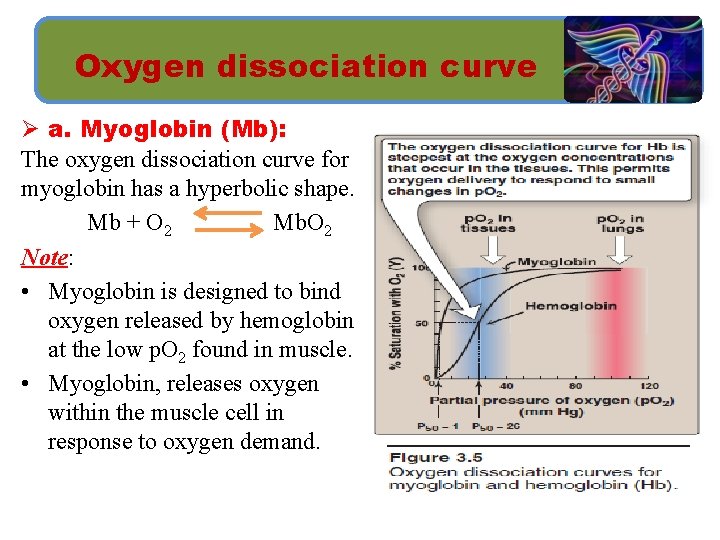
Oxygen dissociation curve Ø a. Myoglobin (Mb): The oxygen dissociation curve for myoglobin has a hyperbolic shape. Mb + O 2 Mb. O 2 Note: • Myoglobin is designed to bind oxygen released by hemoglobin at the low p. O 2 found in muscle. • Myoglobin, releases oxygen within the muscle cell in response to oxygen demand.
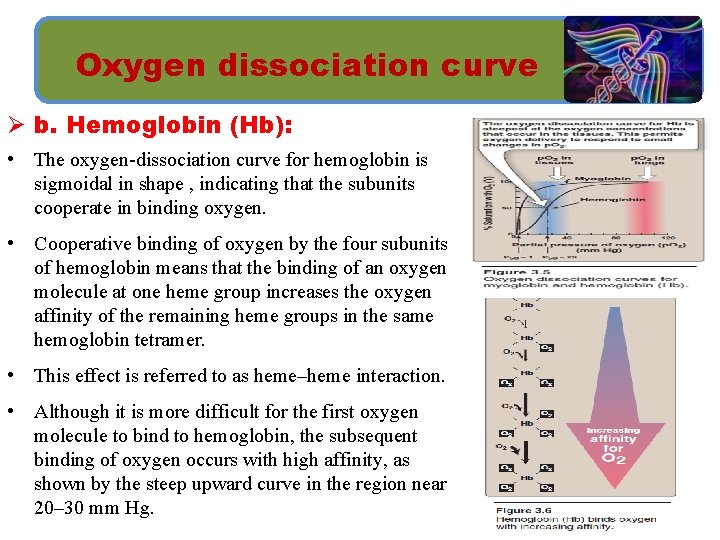
Oxygen dissociation curve Ø b. Hemoglobin (Hb): • The oxygen-dissociation curve for hemoglobin is sigmoidal in shape , indicating that the subunits cooperate in binding oxygen. • Cooperative binding of oxygen by the four subunits of hemoglobin means that the binding of an oxygen molecule at one heme group increases the oxygen affinity of the remaining heme groups in the same hemoglobin tetramer. • This effect is referred to as heme–heme interaction. • Although it is more difficult for the first oxygen molecule to bind to hemoglobin, the subsequent binding of oxygen occurs with high affinity, as shown by the steep upward curve in the region near 20– 30 mm Hg.
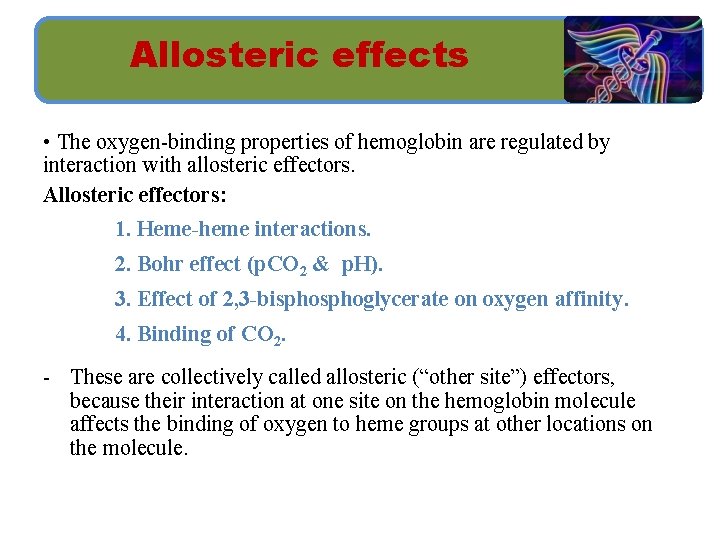
Allosteric effects • The oxygen-binding properties of hemoglobin are regulated by interaction with allosteric effectors. Allosteric effectors: 1. Heme-heme interactions. 2. Bohr effect (p. CO 2 & p. H). 3. Effect of 2, 3 -bisphoglycerate on oxygen affinity. 4. Binding of CO 2. - These are collectively called allosteric (“other site”) effectors, because their interaction at one site on the hemoglobin molecule affects the binding of oxygen to heme groups at other locations on the molecule.
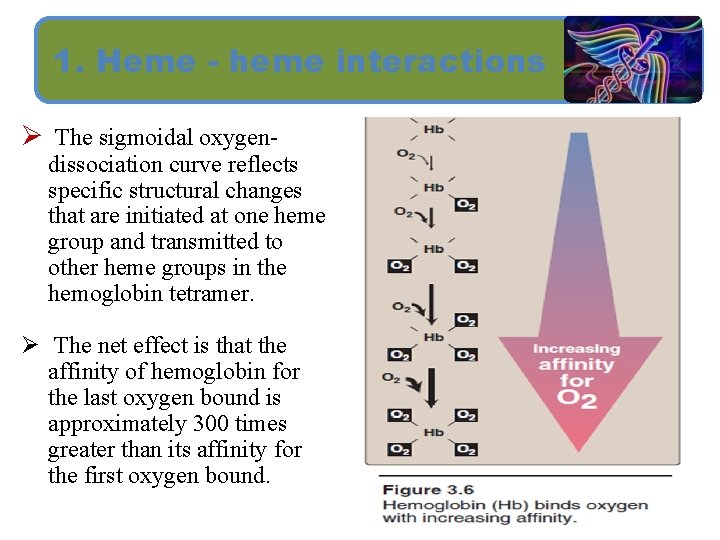
1. Heme - heme interactions Ø The sigmoidal oxygen- dissociation curve reflects specific structural changes that are initiated at one heme group and transmitted to other heme groups in the hemoglobin tetramer. Ø The net effect is that the affinity of hemoglobin for the last oxygen bound is approximately 300 times greater than its affinity for the first oxygen bound.
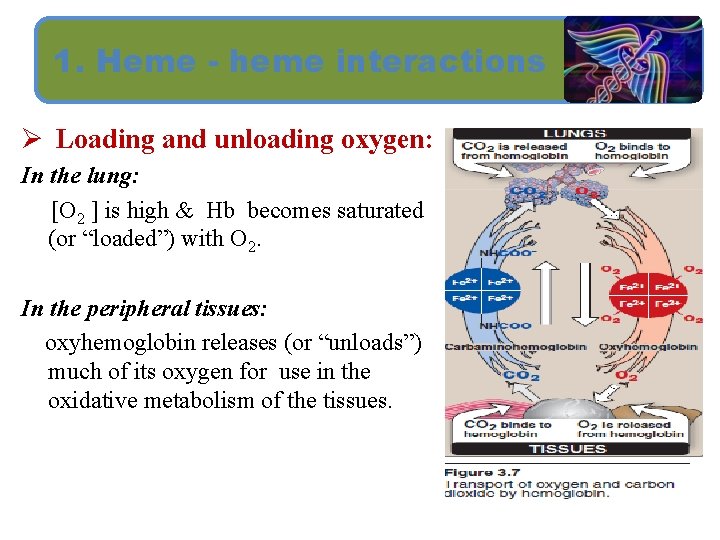
1. Heme - heme interactions Ø Loading and unloading oxygen: In the lung: [O 2 ] is high & Hb becomes saturated (or “loaded”) with O 2. In the peripheral tissues: oxyhemoglobin releases (or “unloads”) much of its oxygen for use in the oxidative metabolism of the tissues.

2. Bohr effect Ø When p. H is lowered, or CO 2 concentration is increased , both result in decreased oxygen affinity of hemoglobin. (both stabilize T state). = Shifts the curve to the right. Ø Raising p. H or low CO 2 concentration results in a greater affinity for oxygen. (both stablize R state). = Shifts the curve to the left.
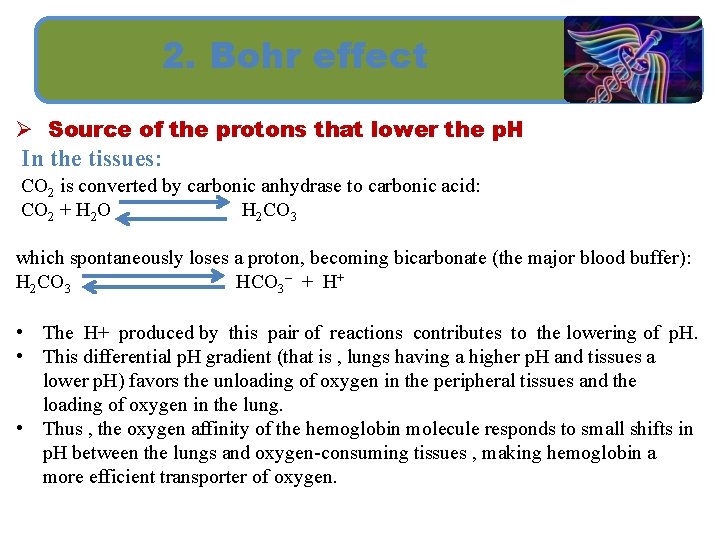
2. Bohr effect Ø Source of the protons that lower the p. H In the tissues: CO 2 is converted by carbonic anhydrase to carbonic acid: CO 2 + H 2 O H 2 CO 3 which spontaneously loses a proton, becoming bicarbonate (the major blood buffer): H 2 CO 3 HCO 3– + H+ • The H+ produced by this pair of reactions contributes to the lowering of p. H. • This differential p. H gradient (that is , lungs having a higher p. H and tissues a lower p. H) favors the unloading of oxygen in the peripheral tissues and the loading of oxygen in the lung. • Thus , the oxygen affinity of the hemoglobin molecule responds to small shifts in p. H between the lungs and oxygen-consuming tissues , making hemoglobin a more efficient transporter of oxygen.
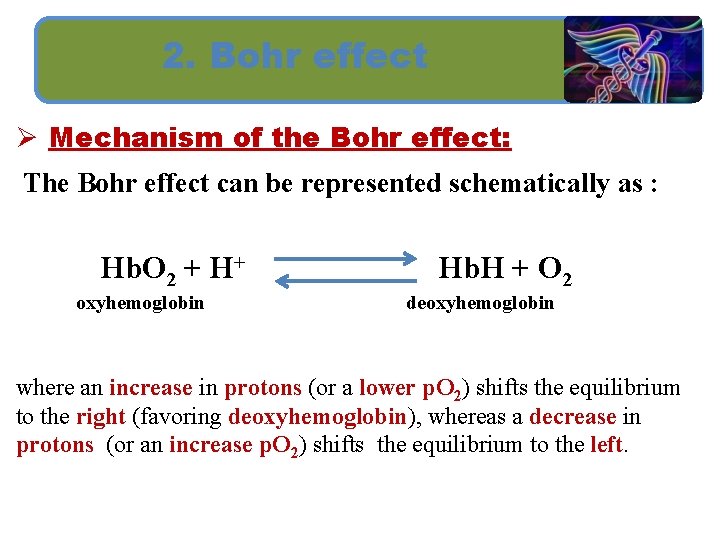
2. Bohr effect Ø Mechanism of the Bohr effect: The Bohr effect can be represented schematically as : Hb. O 2 + H+ oxyhemoglobin Hb. H + O 2 deoxyhemoglobin where an increase in protons (or a lower p. O 2) shifts the equilibrium to the right (favoring deoxyhemoglobin), whereas a decrease in protons (or an increase p. O 2) shifts the equilibrium to the left.
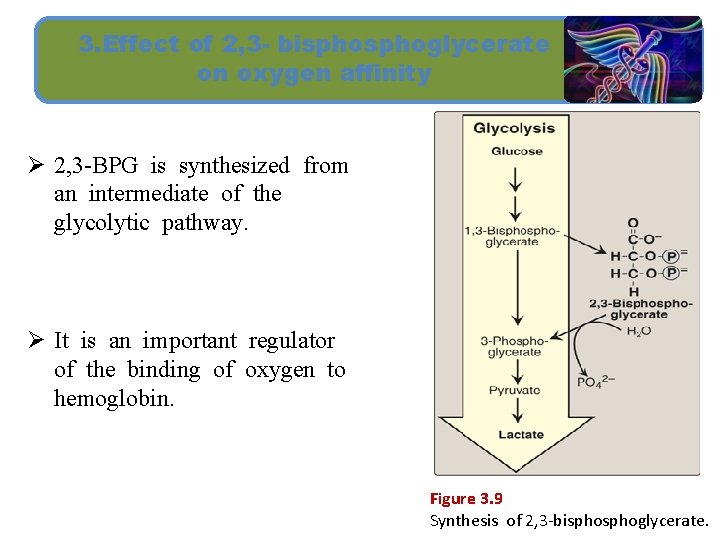
3. Effect of 2, 3 - bisphoglycerate on oxygen affinity Ø 2, 3 -BPG is synthesized from an intermediate of the glycolytic pathway. Ø It is an important regulator of the binding of oxygen to hemoglobin. Figure 3. 9 Synthesis of 2, 3 -bisphoglycerate.
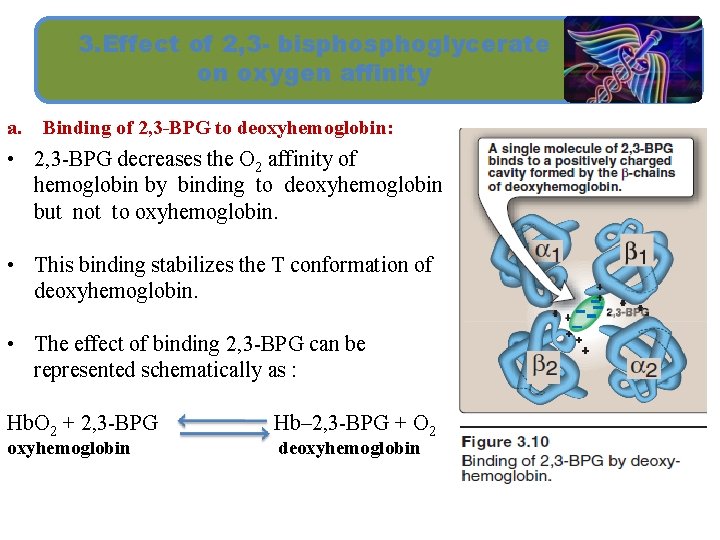
3. Effect of 2, 3 - bisphoglycerate on oxygen affinity a. Binding of 2, 3 -BPG to deoxyhemoglobin: • 2, 3 -BPG decreases the O 2 affinity of hemoglobin by binding to deoxyhemoglobin but not to oxyhemoglobin. • This binding stabilizes the T conformation of deoxyhemoglobin. • The effect of binding 2, 3 -BPG can be represented schematically as : Hb. O 2 + 2, 3 -BPG oxyhemoglobin Hb– 2, 3 -BPG + O 2 deoxyhemoglobin
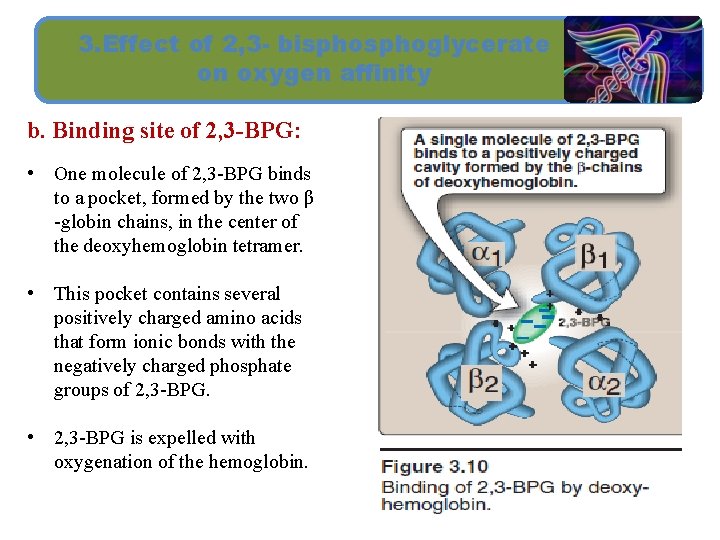
3. Effect of 2, 3 - bisphoglycerate on oxygen affinity b. Binding site of 2, 3 -BPG: • One molecule of 2, 3 -BPG binds to a pocket, formed by the two β -globin chains, in the center of the deoxyhemoglobin tetramer. • This pocket contains several positively charged amino acids that form ionic bonds with the negatively charged phosphate groups of 2, 3 -BPG. • 2, 3 -BPG is expelled with oxygenation of the hemoglobin.
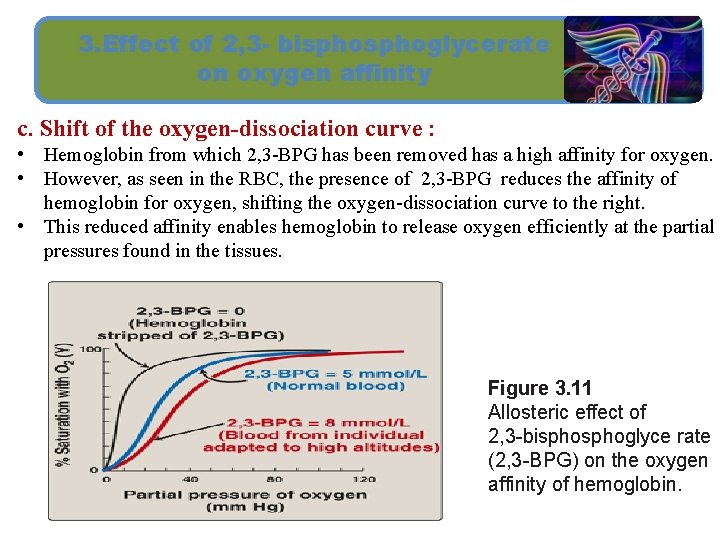
3. Effect of 2, 3 - bisphoglycerate on oxygen affinity c. Shift of the oxygen-dissociation curve : • Hemoglobin from which 2, 3 -BPG has been removed has a high affinity for oxygen. • However, as seen in the RBC, the presence of 2, 3 -BPG reduces the affinity of hemoglobin for oxygen, shifting the oxygen-dissociation curve to the right. • This reduced affinity enables hemoglobin to release oxygen efficiently at the partial pressures found in the tissues. Figure 3. 11 Allosteric effect of 2, 3 -bisphoglyce rate (2, 3 -BPG) on the oxygen affinity of hemoglobin.
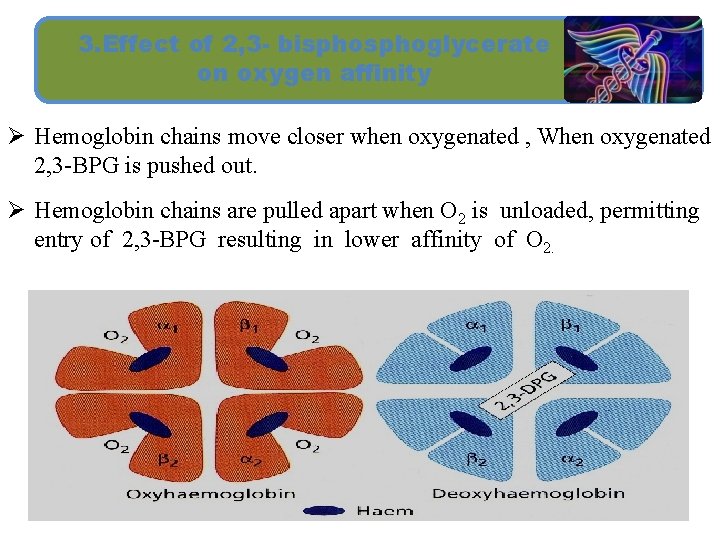
3. Effect of 2, 3 - bisphoglycerate on oxygen affinity Ø Hemoglobin chains move closer when oxygenated , When oxygenated 2, 3 -BPG is pushed out. Ø Hemoglobin chains are pulled apart when O 2 is unloaded, permitting entry of 2, 3 -BPG resulting in lower affinity of O 2.
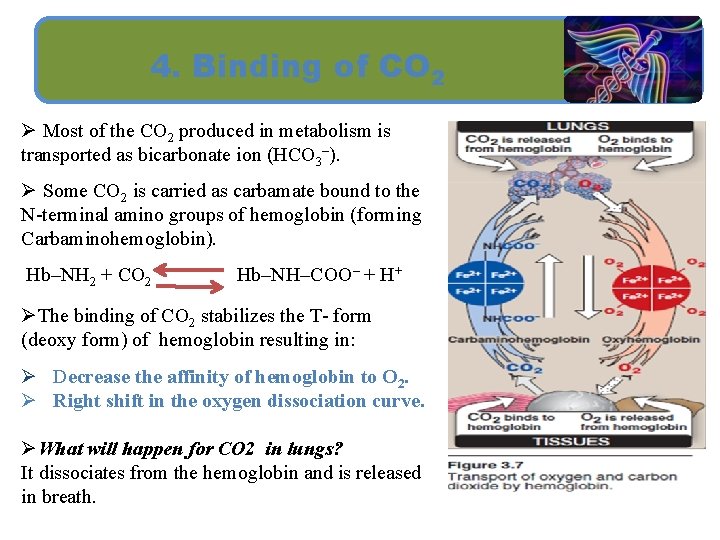
4. Binding of CO 2 Ø Most of the CO 2 produced in metabolism is transported as bicarbonate ion (HCO 3–). Ø Some CO 2 is carried as carbamate bound to the N-terminal amino groups of hemoglobin (forming Carbaminohemoglobin). Hb–NH 2 + CO 2 Hb–NH–COO– + H+ ØThe binding of CO 2 stabilizes the T- form (deoxy form) of hemoglobin resulting in: Ø Decrease the affinity of hemoglobin to O 2. Ø Right shift in the oxygen dissociation curve. ØWhat will happen for CO 2 in lungs? It dissociates from the hemoglobin and is released in breath.
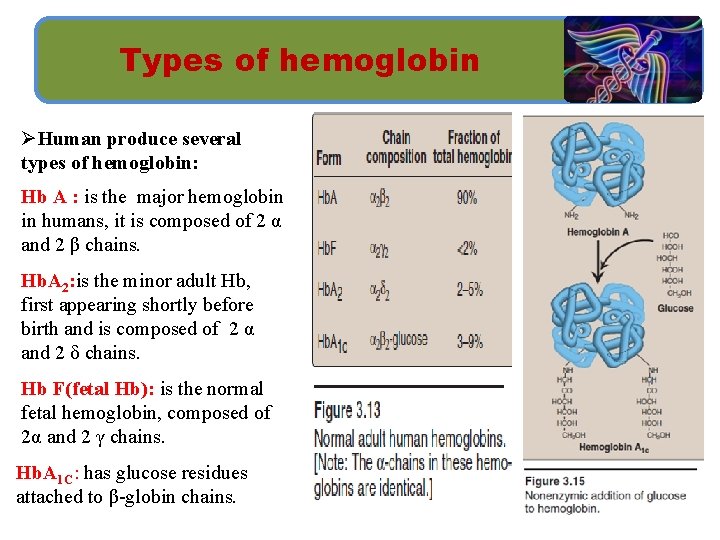
Types of hemoglobin ØHuman produce several types of hemoglobin: Hb A : is the major hemoglobin in humans, it is composed of 2 α and 2 β chains. Hb. A 2: is the minor adult Hb, first appearing shortly before birth and is composed of 2 α and 2 δ chains. Hb F(fetal Hb): is the normal fetal hemoglobin, composed of 2α and 2 γ chains. Hb. A 1 C: has glucose residues attached to β-globin chains.


SUMMARY ■ Myoglobin is monomeric; hemoglobin is a tetramer of two subunit types (α 2β 2 in Hb. A). Despite having different primary structures, myoglobin and the subunits of hemoglobin have nearly identical secondary and tertiary structures. ■ Heme is a cyclic tetrapyrrole has a central Fe 2+ linked to all four nitrogen atoms of the heme, to histidine F 8, and, in oxy. Mb and oxy. Hb, also to O 2. ■ The deoxy form of Hb is called the “T, ” or taut (tense) conformation and it is the low-oxygen-affinity form of Hb. The binding of O 2 to Hb causes rupture of some of the ionic and hydrogen bonds, and movement of the dimers and this leads to a structure called the “R, ” or relaxed conformation which is the high-oxygen affinity form of Hb.
SUMMARY ■ The oxygen-dissociation curve for Hb is sigmoidal in shape (in contrast to that of myoglobin, which is hyperbolic), indicating that the subunits cooperate in binding O 2. ■ Cooperative binding of O 2 by the four subunits of Hb means that the binding of an O 2 molecule at one heme group increases the oxygen affinity of the remaining heme groups in the same Hb molecule. ■ Hb’s ability to bind O 2 reversibly is affected by the partial pressure of O 2 (p. O 2) (through heme-heme interactions), the p. H of the environment, the partial pressure of CO 2 (p. CO 2), and the availability of 2, 3 -bisphoglycerate (2, 3 -BPG).

Home work Why does CO (carbon monoxide) considered as severe toxic gas? ? ? 39

Thank you
- Slides: 40