Hemoglobin and Red Blood Cells Hemoglobin quaternary structure

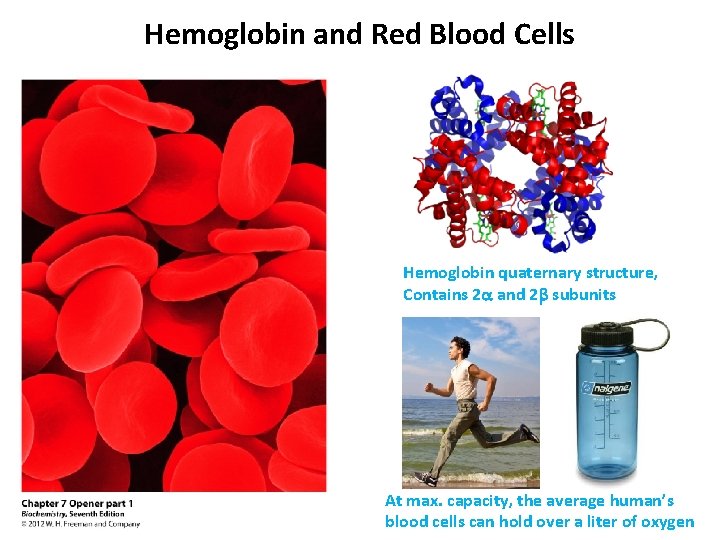
Hemoglobin and Red Blood Cells Hemoglobin quaternary structure, Contains 2 a and 2 b subunits At max. capacity, the average human’s blood cells can hold over a liter of oxygen
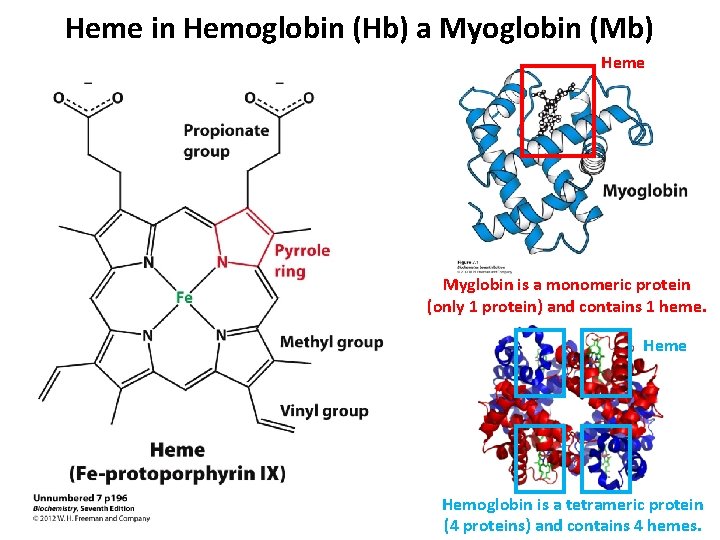
Heme in Hemoglobin (Hb) a Myoglobin (Mb) Heme Myglobin is a monomeric protein (only 1 protein) and contains 1 heme. Heme Hemoglobin is a tetrameric protein (4 proteins) and contains 4 hemes.
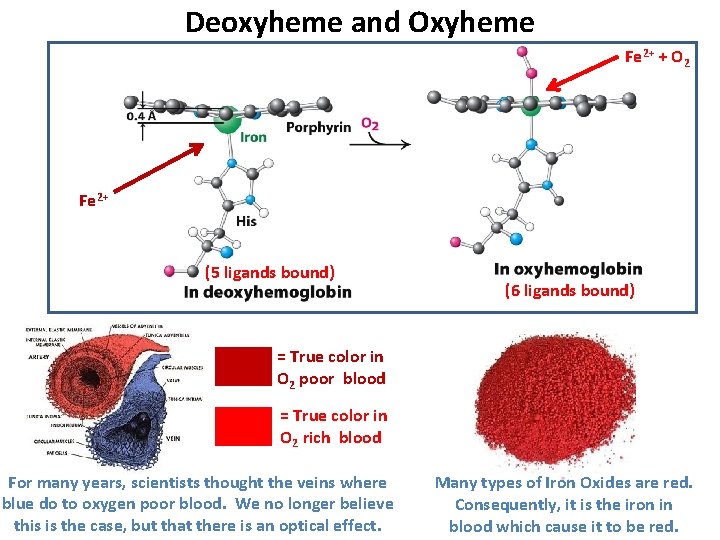
Deoxyheme and Oxyheme Fe 2+ + O 2 Fe 2+ (5 ligands bound) (6 ligands bound) = True color in O 2 poor blood = True color in O 2 rich blood For many years, scientists thought the veins where blue do to oxygen poor blood. We no longer believe this is the case, but that there is an optical effect. Many types of Iron Oxides are red. Consequently, it is the iron in blood which cause it to be red.
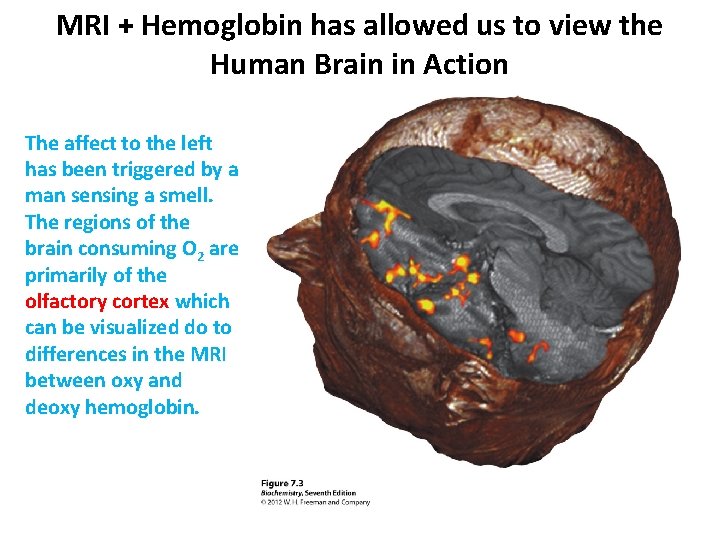
MRI + Hemoglobin has allowed us to view the Human Brain in Action The affect to the left has been triggered by a man sensing a smell. The regions of the brain consuming O 2 are primarily of the olfactory cortex which can be visualized do to differences in the MRI between oxy and deoxy hemoglobin.
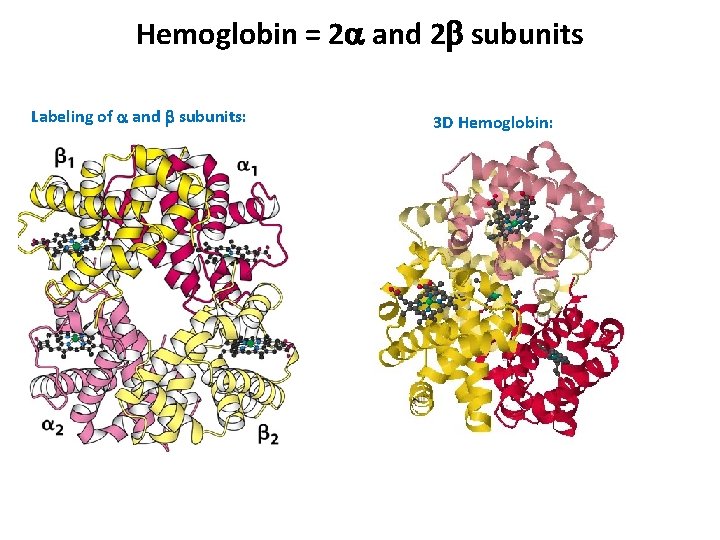
Hemoglobin = 2 a and 2 b subunits Labeling of a and b subunits: 3 D Hemoglobin:
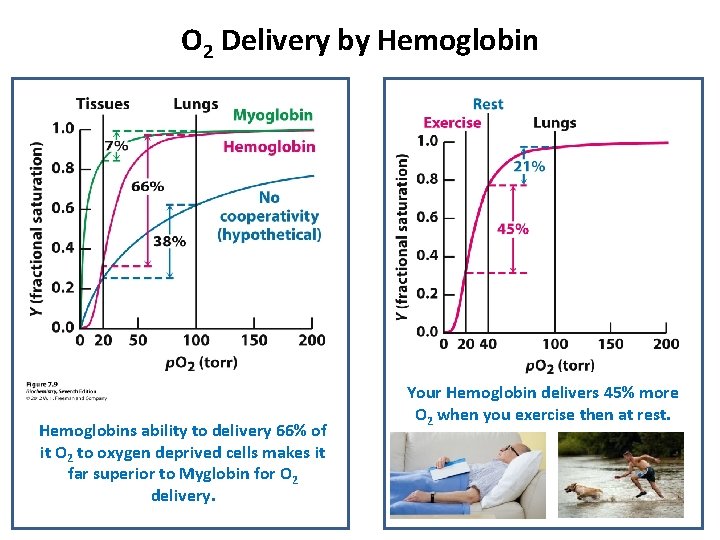
O 2 Delivery by Hemoglobins ability to delivery 66% of it O 2 to oxygen deprived cells makes it far superior to Myglobin for O 2 delivery. Your Hemoglobin delivers 45% more O 2 when you exercise then at rest.
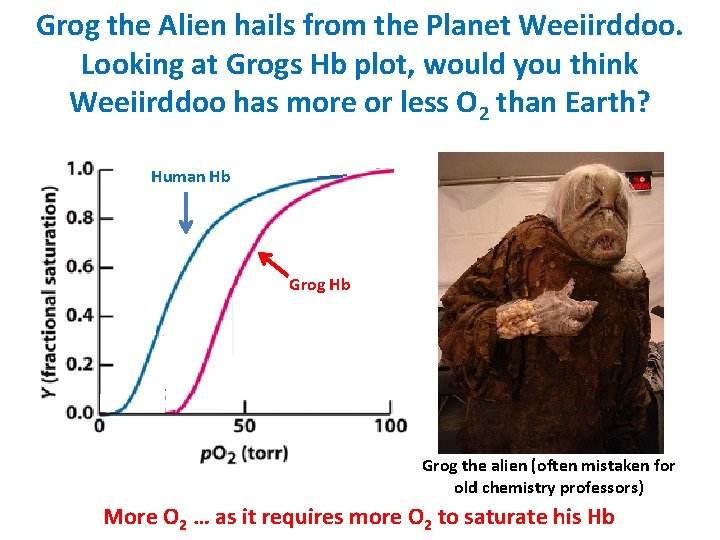
Grog the Alien hails from the Planet Weeiirddoo. Looking at Grogs Hb plot, would you think Weeiirddoo has more or less O 2 than Earth? Human Hb Grog the alien (often mistaken for old chemistry professors) More O 2 … as it requires more O 2 to saturate his Hb
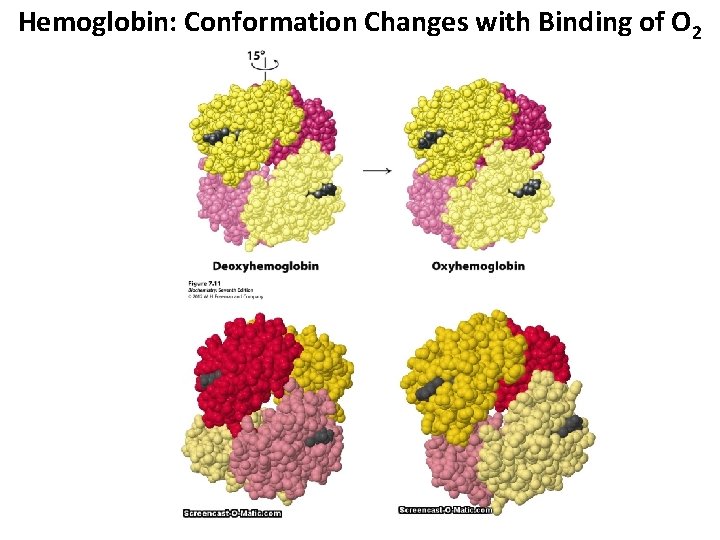
Hemoglobin: Conformation Changes with Binding of O 2
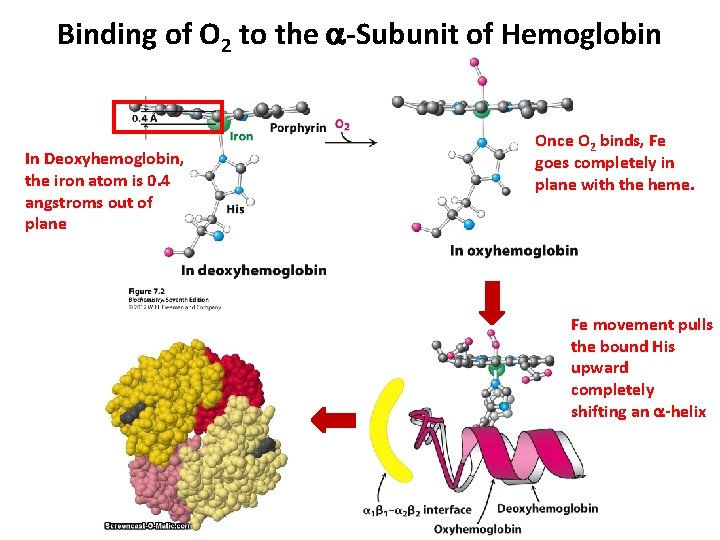
Binding of O 2 to the a-Subunit of Hemoglobin In Deoxyhemoglobin, the iron atom is 0. 4 angstroms out of plane Once O 2 binds, Fe goes completely in plane with the heme. Fe movement pulls the bound His upward completely shifting an a-helix
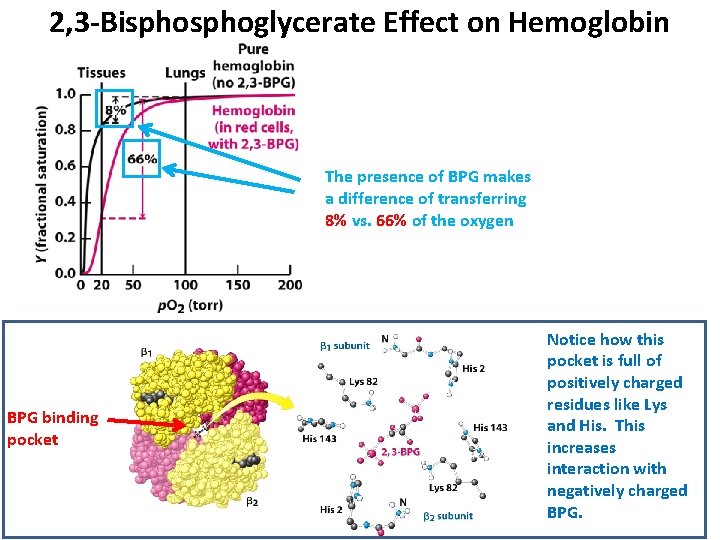
2, 3 -Bisphoglycerate Effect on Hemoglobin The presence of BPG makes a difference of transferring 8% vs. 66% of the oxygen BPG binding pocket Notice how this pocket is full of positively charged residues like Lys and His. This increases interaction with negatively charged BPG.
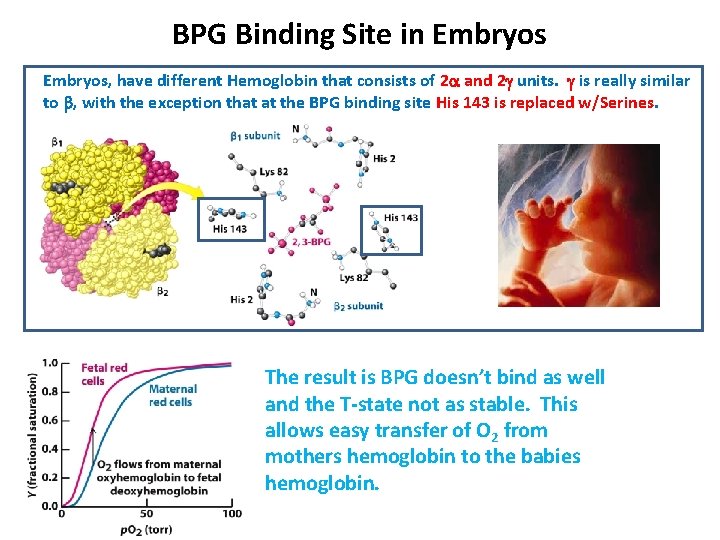
BPG Binding Site in Embryos, have different Hemoglobin that consists of 2 a and 2 g units. g is really similar to b, with the exception that at the BPG binding site His 143 is replaced w/Serines. The result is BPG doesn’t bind as well and the T-state not as stable. This allows easy transfer of O 2 from mothers hemoglobin to the babies hemoglobin.

Carbon Monoxide Poisoning Due to similar shape, CO binds like O 2 to Hemoglobin: Why CO is deadly: 1. Binds Fe 2+ 1000 x stronger than O 2 (out competes sites on Hb) 2. Forces Hb into R-state (BPG can’t bind and O 2 bound can’t be released well) 3. The result of above is the suffocation of brain cells that leads to death Roughly 2500 people die of CO each year: 2000 are suicides. Treatment: expose to pure O 2
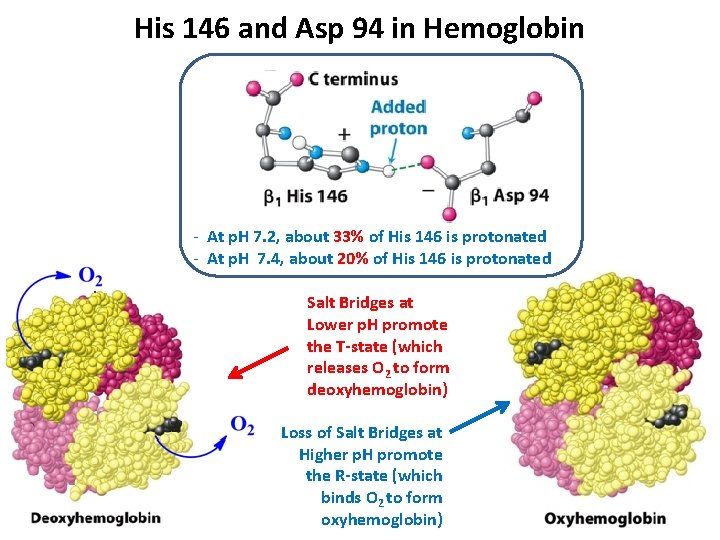
His 146 and Asp 94 in Hemoglobin - At p. H 7. 2, about 33% of His 146 is protonated - At p. H 7. 4, about 20% of His 146 is protonated Salt Bridges at Lower p. H promote the T-state (which releases O 2 to form deoxyhemoglobin) Loss of Salt Bridges at Higher p. H promote the R-state (which binds O 2 to form oxyhemoglobin)
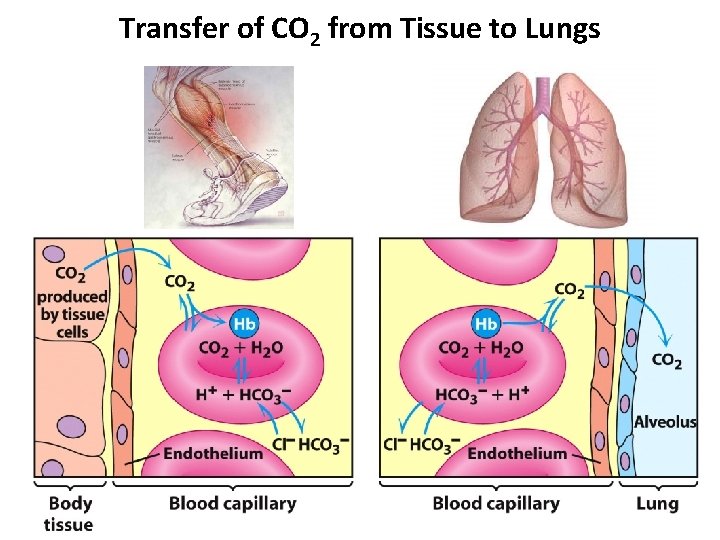
Transfer of CO 2 from Tissue to Lungs
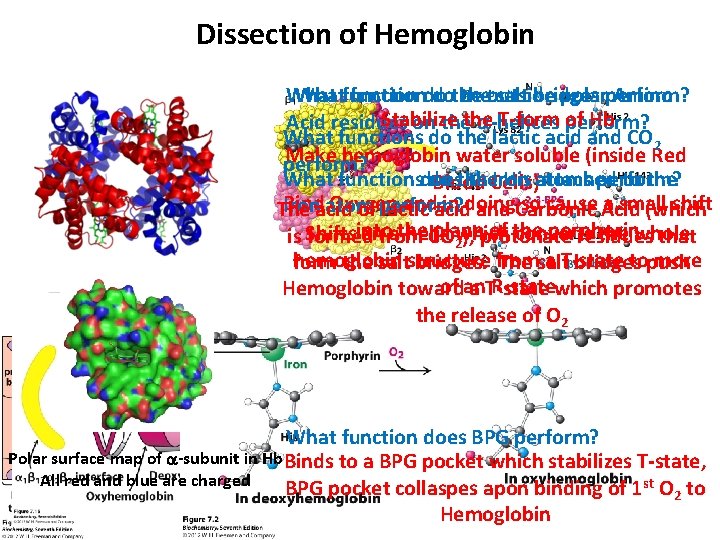
Dissection of Hemoglobin Whatfunctiondo dothe theoutside salt bridges polarperform? Amino Stabilize T-form perform? of Hb Acid residues on thethe a-helices What functions do the lactic acid and CO 2 Make hemoglobin water soluble (inside Red perform? What function the. Cells) His atoms attached to the functionsdoes do the Iron perform? Blood Bind Oxygen andacid in doing so cause Acid a small shift Ironacid atom The of perform? lactic and Carbonic (which into the. CO plane of the porphorin anfrom a-helix which converts the whole is Shifts formed 2), protonate residues that hemoglobin a T-state topush more form the saltstructure bridges. from The salt bridges of an R-statewhich promotes Hemoglobin toward a T-state the release of O 2 What function does BPG perform? Polar surface map of a-subunit in Hb Binds to a BPG pocket which stabilizes T-state, All red and blue are charged BPG pocket collaspes apon binding of 1 st O 2 to Hemoglobin
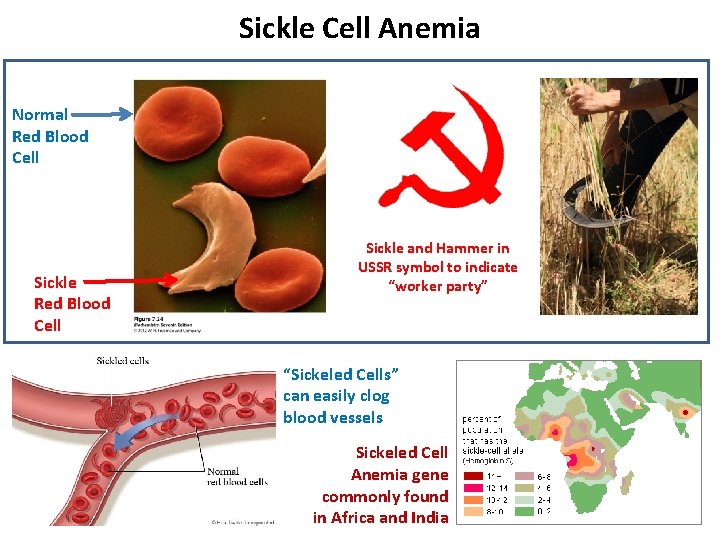
Sickle Cell Anemia Normal Red Blood Cell Sickle and Hammer in USSR symbol to indicate “worker party” “Sickeled Cells” can easily clog blood vessels Sickeled Cell Anemia gene commonly found in Africa and India
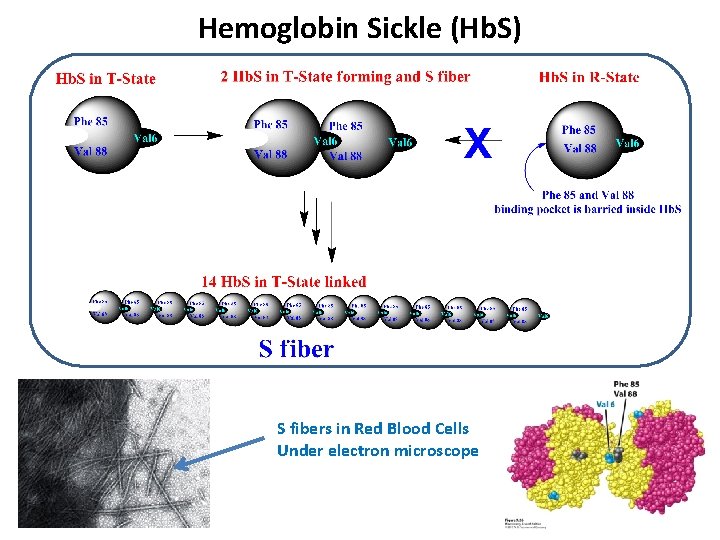
Hemoglobin Sickle (Hb. S) S fibers in Red Blood Cells Under electron microscope
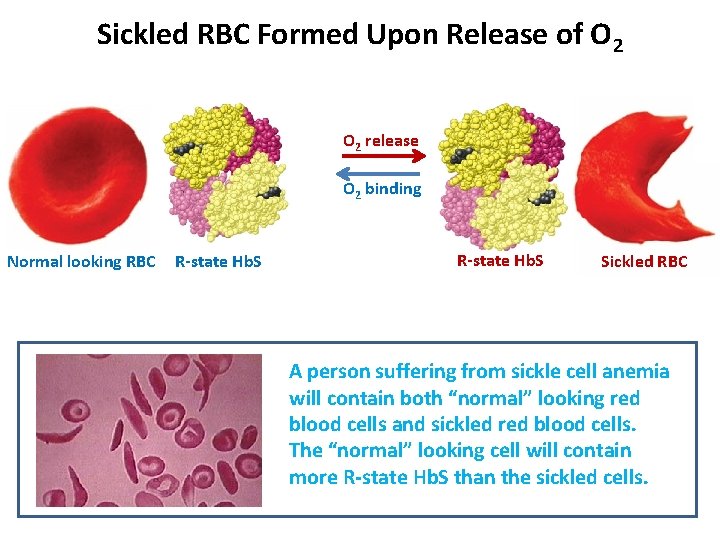
Sickled RBC Formed Upon Release of O 2 release O 2 binding Normal looking RBC R-state Hb. S Sickled RBC A person suffering from sickle cell anemia will contain both “normal” looking red blood cells and sickled red blood cells. The “normal” looking cell will contain more R-state Hb. S than the sickled cells.
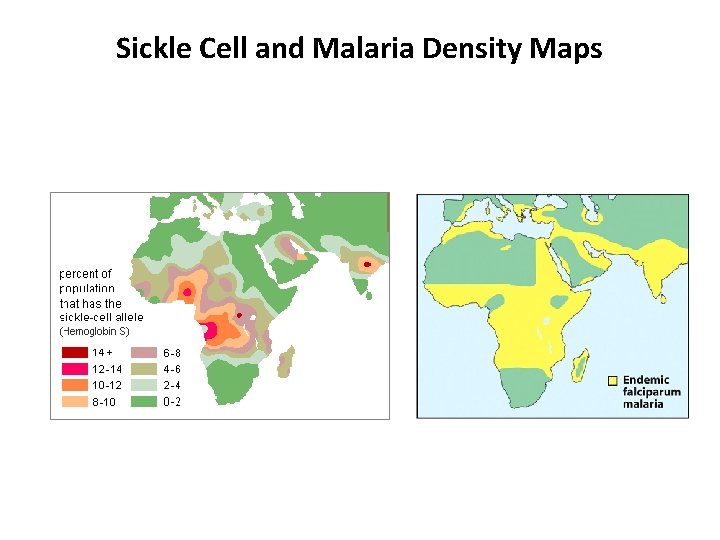
Sickle Cell and Malaria Density Maps
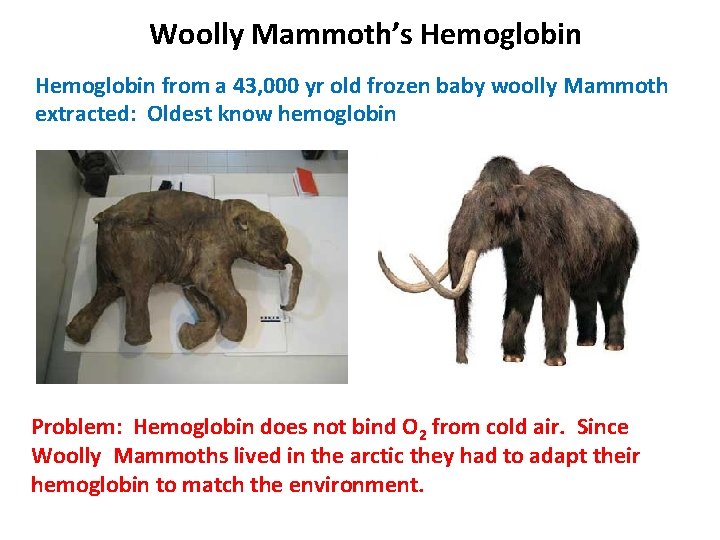
Woolly Mammoth’s Hemoglobin from a 43, 000 yr old frozen baby woolly Mammoth extracted: Oldest know hemoglobin Problem: Hemoglobin does not bind O 2 from cold air. Since Woolly Mammoths lived in the arctic they had to adapt their hemoglobin to match the environment.
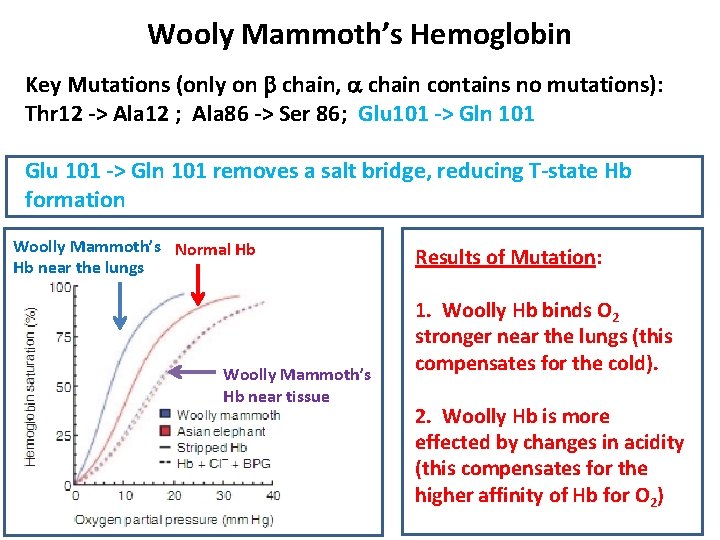
Wooly Mammoth’s Hemoglobin Key Mutations (only on b chain, a chain contains no mutations): Thr 12 -> Ala 12 ; Ala 86 -> Ser 86; Glu 101 -> Gln 101 removes a salt bridge, reducing T-state Hb formation Woolly Mammoth’s Normal Hb Hb near the lungs Woolly Mammoth’s Hb near tissue Results of Mutation: 1. Woolly Hb binds O 2 stronger near the lungs (this compensates for the cold). 2. Woolly Hb is more effected by changes in acidity (this compensates for the higher affinity of Hb for O 2)
- Slides: 23