HEMA DIAGNOSTIC SYSTEMS HDS DIAGNOSTIC SOLUTIONS FOR A























- Slides: 44

HEMA DIAGNOSTIC SYSTEMS (HDS) …DIAGNOSTIC SOLUTIONS FOR A CHANGING WORLD™… Dr. Paul D Slowey Executive Vice-President Hema Diagnostic Systems May 2006 North Bay Village, Florida USA Saõ Paulo, Brazil Panama City, Panama

MISSION AND VISION STATEMENTS Mission Statement l To provide the highest quality rapid diagnostic testing devices to the global market place in a cost effective manner Vision Statement l l l To be recognized globally as a leading high quality developer and manufacturer of rapid diagnostic assays To respond to the needs of its customers by producing a continual pipeline of innovative point-of-care tests To be focused on customer satisfaction and innovate as market forces dictate

BRIEF HISTORY OF HDS § December, 2000: Founded as Hema Diagnostic Systems (“HDS”) in Miami Beach Florida USA § July 2002: HDS Panama founded in Panama City, Panama for assembly/packaging § October 2003: Awarded patents for the Rapid 1 -2 -3® Hema EZ technology platform § January 2004: Licensed and subsequently acquired proprietary technology for tuberculosis from European Company § March 2004: Filed patents on the Rapid 1 -2 -3® Hema Express technology platform § November 2004: Established new R&D facility (North Bay Village, Florida) § November 2005: Opened new HDS branch office in São Paulo, Brazil § February 2006: Joined Global Business Coalition (GBC)

SUMMARY OF HDS PRODUCTS / SERVICES

HDS offers high quality, reliable assays that address the global medical community’s need for simple, fast, and affordable rapid testing for infectious diseases including HIV/AIDS, tuberculosis, malaria, and others.

HDS offers unique proprietary and patented delivery systems incorporating high-quality test strips, manufactured under US GMP conditions, creating a system of rapid diagnostic assays unlike that of any other company.

RAPID TESTING

WHAT IS RAPID TESTING? Ø Ø Ø Ø A rapid test is generally conducted in the field or clinic setting, outside of a laboratory Tests are usually based on the principles of (lateral flow) immunochromatography or hemaglutination Performed typically on, whole blood, urine or oral fluid Test results available in 10 -20 minutes instead of days High degree of sensitivity/specificity Require limited training, simple to administer Require little or no equipment

HDS RAPID TESTING TECHNOLOGIES Rapid 1 -2 -3® Hema immunoassays include individually color-coded tests for: --HIV 1 / 2 --Tuberculosis (TB) --Malaria (pf, pf/pv/p total) --Syphilis

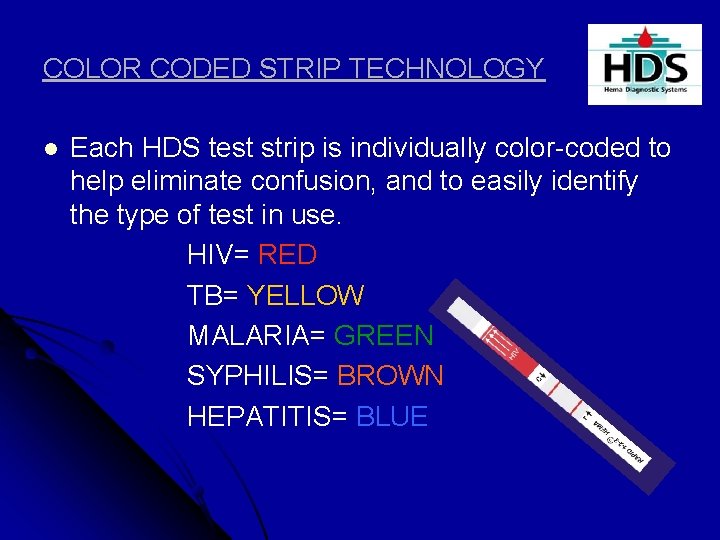
COLOR CODED STRIP TECHNOLOGY l Each HDS test strip is individually color-coded to help eliminate confusion, and to easily identify the type of test in use. HIV= RED TB= YELLOW MALARIA= GREEN SYPHILIS= BROWN HEPATITIS= BLUE

PACKAGING Color coding on (foil) packaging matches color coding of test strips in the various product formats

HDS RAPID TESTING PLATFORMS HDS Rapid Testing platforms include: Ø RAPID 1 -2 -3® HEMA EXPRESS™ Ø RAPID 1 -2 -3® HEMA EZ™ Ø RAPID 1 -2 -3® HEMA DIPSTICK STRIPS

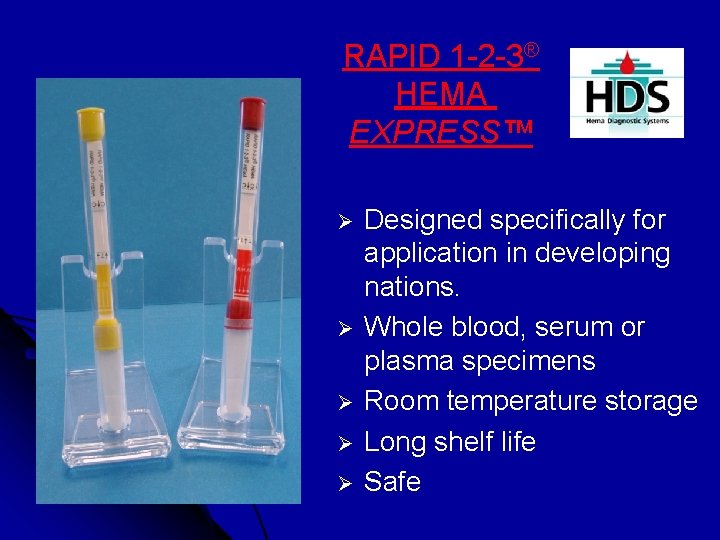
RAPID 1 -2 -3® HEMA EXPRESS™ Ø Ø Ø Designed specifically for application in developing nations. Whole blood, serum or plasma specimens Room temperature storage Long shelf life Safe

RAPID 1 -2 -3® HEMA EXPRESS™ The EXPRESS® device is an easy-to-use, accurate, low cost, rapid testing platform with a small sample requirement (10 -30 μL, fingerstick blood). All components are safe and simple to handle. HOW IT WORKS: Remove protective cover from EXPRESS ® device, exposing the Ø sample pad.

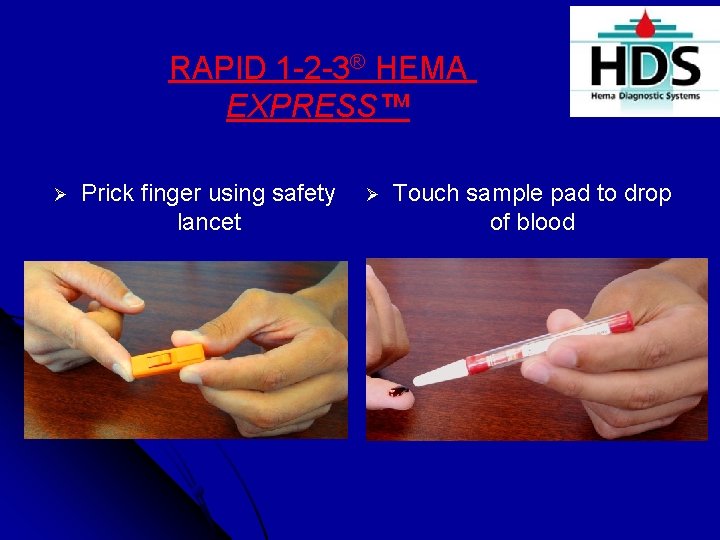
RAPID 1 -2 -3® HEMA EXPRESS™ Ø Prick finger using safety lancet Ø Touch sample pad to drop of blood

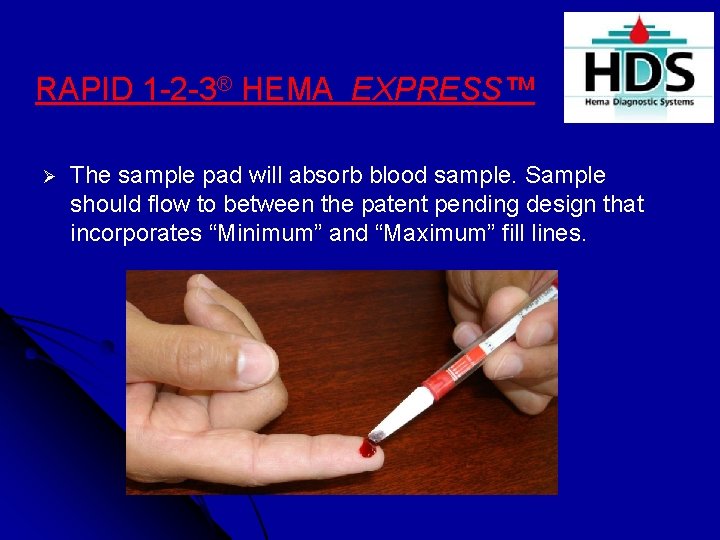
RAPID 1 -2 -3® HEMA EXPRESS™ Ø The sample pad will absorb blood sample. Sample should flow to between the patent pending design that incorporates “Minimum” and “Maximum” fill lines.

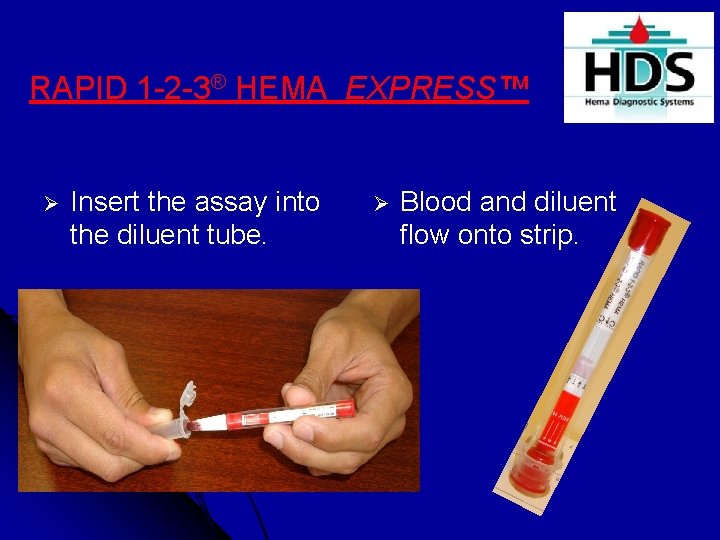
RAPID 1 -2 -3® HEMA EXPRESS™ Ø Insert the assay into the diluent tube. Ø Blood and diluent flow onto strip.

RAPID 1 -2 -3® HEMA EXPRESS™ Ø Within a few minutes a Control line appears indicating the test is functioning correctly.

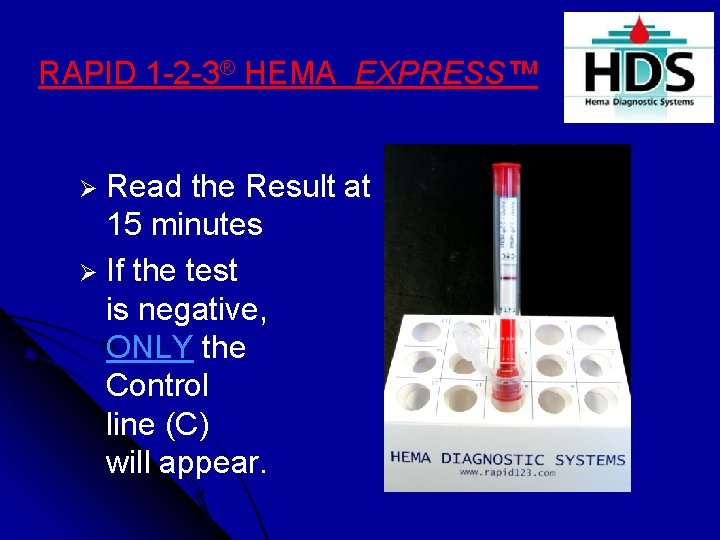
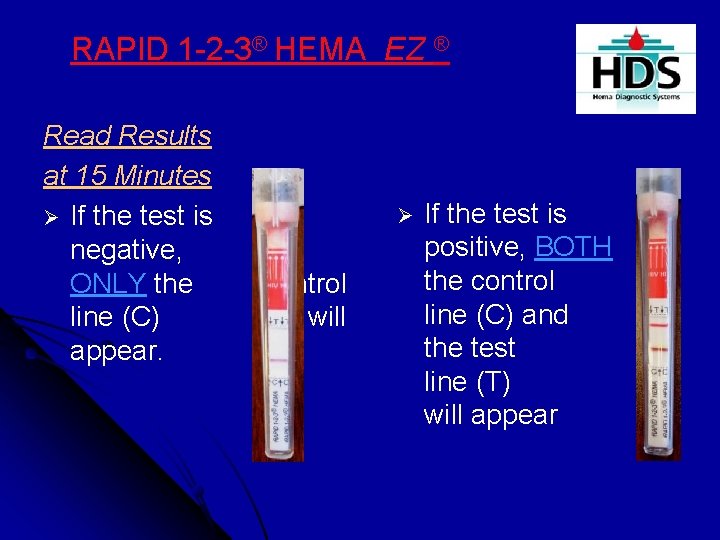
RAPID 1 -2 -3® HEMA EXPRESS™ Ø Read the Result at 15 minutes Ø If the test is negative, ONLY the Control line (C) will appear.

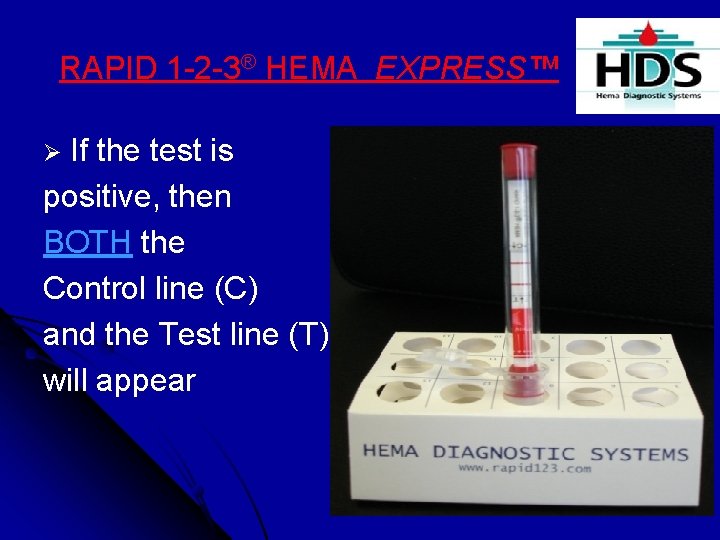
RAPID 1 -2 -3® HEMA EXPRESS™ Ø If the test is positive, then BOTH the Control line (C) and the Test line (T) will appear

RAPID 1 -2 -3® HEMA EZ ® PLATFORM Ø Ø Ø Designed to meet the requirements of the Global market, including the United States, EU and Japan A simple and reproducible method of testing in point of care situations such as doctors offices, mobile clinics, outpatient centers Currently being considered for over-the-counter use in a number of countries around the world.

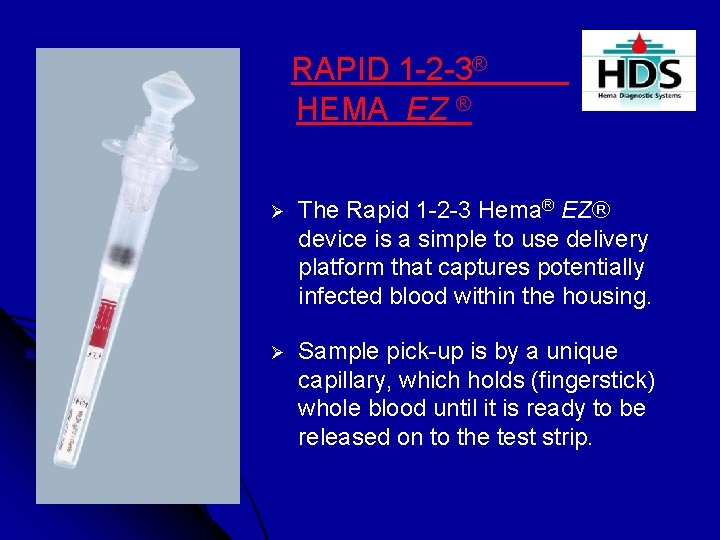
RAPID 1 -2 -3® HEMA EZ ® Ø The Rapid 1 -2 -3 Hema® EZ® device is a simple to use delivery platform that captures potentially infected blood within the housing. Ø Sample pick-up is by a unique capillary, which holds (fingerstick) whole blood until it is ready to be released on to the test strip.

RAPID 1 -2 -3® HEMA EZ ® HOW IT WORKS Ø Prick finger with safety lancet Ø Touch the tip of the sampler to the drop of blood. Blood will automatically be drawn up by capillary action into the sampler

RAPID 1 -2 -3®HEMA EZ ® l Place sampler containing the blood collected into the lower housing.

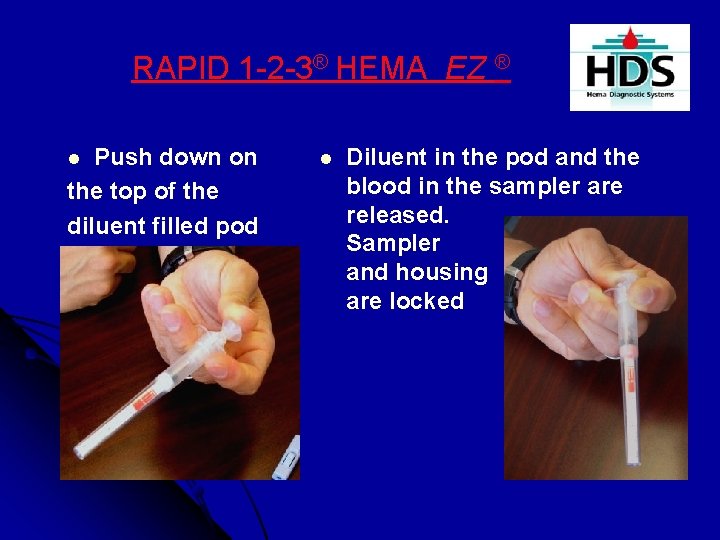
RAPID 1 -2 -3® HEMA EZ ® Push down on the top of the diluent filled pod l l Diluent in the pod and the blood in the sampler are released. Sampler and housing are locked in place.

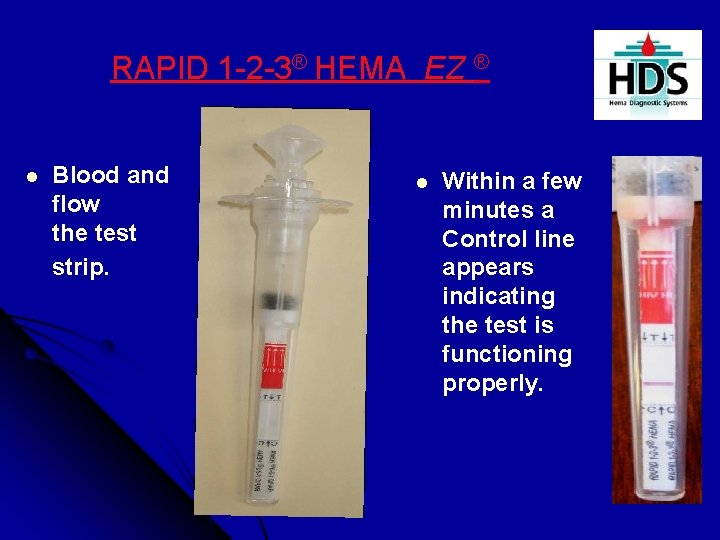
RAPID 1 -2 -3® HEMA EZ ® l Blood and flow the test strip. diluent down onto l Within a few minutes a Control line appears indicating the test is functioning properly.

RAPID 1 -2 -3® HEMA EZ ® Read Results at 15 Minutes Ø If the test is negative, ONLY the line (C) appear. Ø control will If the test is positive, BOTH the control line (C) and the test line (T) will appear

HDS DIPSTICK FORMAT ASSAYS Each assay consists of: l Desiccated canister (containing 25, 50, or 100 test strips) l Vial with diluent buffer l Safety lancets l Blood loops l OEM packaging available (optional) l Sample trays (upon request)

HIV CLINICAL DATA DIPSTICK AND EXPRESS™ DATA

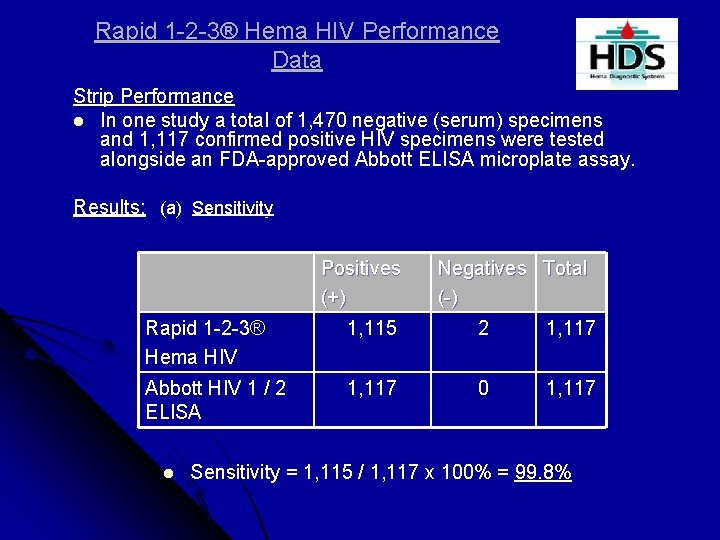
Rapid 1 -2 -3® Hema HIV Performance Data Strip Performance l In one study a total of 1, 470 negative (serum) specimens and 1, 117 confirmed positive HIV specimens were tested alongside an FDA-approved Abbott ELISA microplate assay. Results: (a) Sensitivity Positives (+) Negatives Total (-) Rapid 1 -2 -3® Hema HIV 1, 115 2 1, 117 Abbott HIV 1 / 2 ELISA 1, 117 0 1, 117 l Sensitivity = 1, 115 / 1, 117 x 100% = 99. 8%

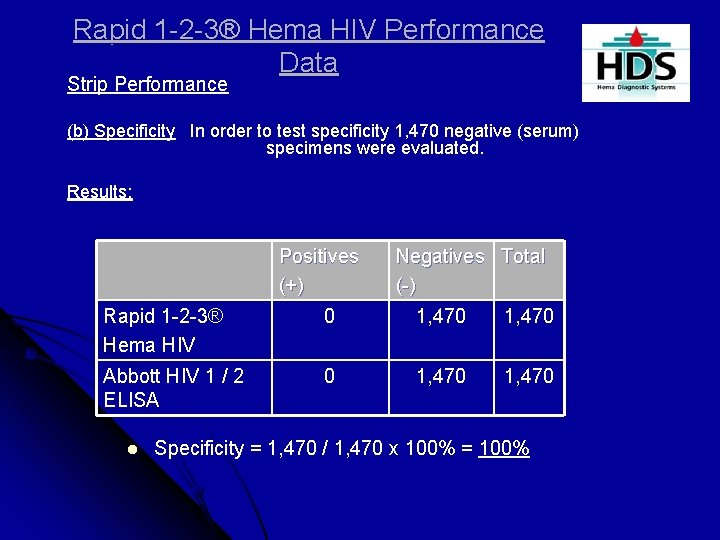
Rapid 1 -2 -3® Hema HIV Performance Data Strip Performance (b) Specificity In order to test specificity 1, 470 negative (serum) specimens were evaluated. Results: Positives (+) Negatives Total (-) Rapid 1 -2 -3® Hema HIV 0 1, 470 Abbott HIV 1 / 2 ELISA 0 1, 470 l Specificity = 1, 470 / 1, 470 x 100% = 100%

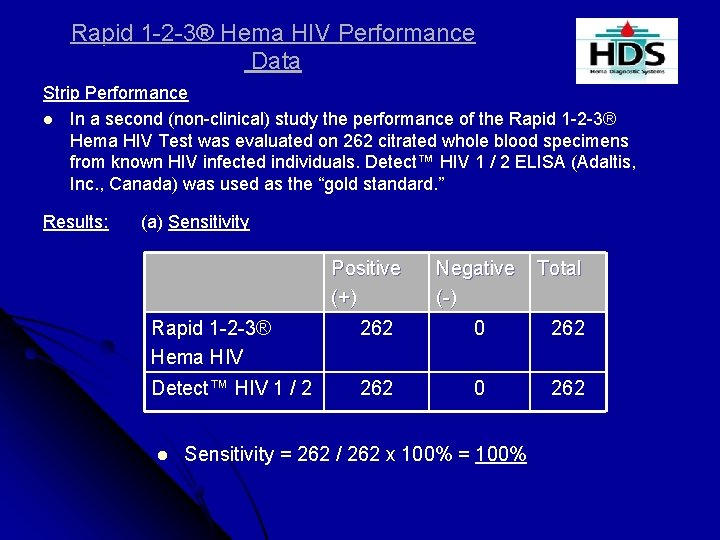
Rapid 1 -2 -3® Hema HIV Performance Data Strip Performance l In a second (non-clinical) study the performance of the Rapid 1 -2 -3® Hema HIV Test was evaluated on 262 citrated whole blood specimens from known HIV infected individuals. Detect™ HIV 1 / 2 ELISA (Adaltis, Inc. , Canada) was used as the “gold standard. ” Results: (a) Sensitivity Positive (+) Negative (-) Total Rapid 1 -2 -3® Hema HIV 262 0 262 Detect™ HIV 1 / 2 262 0 262 l Sensitivity = 262 / 262 x 100% = 100%

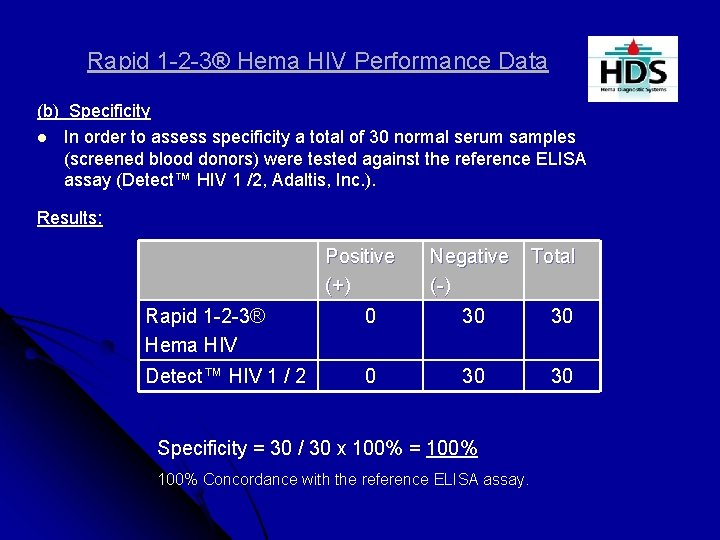
Rapid 1 -2 -3® Hema HIV Performance Data (b) Specificity l In order to assess specificity a total of 30 normal serum samples (screened blood donors) were tested against the reference ELISA assay (Detect™ HIV 1 /2, Adaltis, Inc. ). Results: Positive (+) Negative (-) Total Rapid 1 -2 -3® Hema HIV 0 30 30 Detect™ HIV 1 / 2 0 30 30 Specificity = 30 / 30 x 100% = 100% Concordance with the reference ELISA assay.

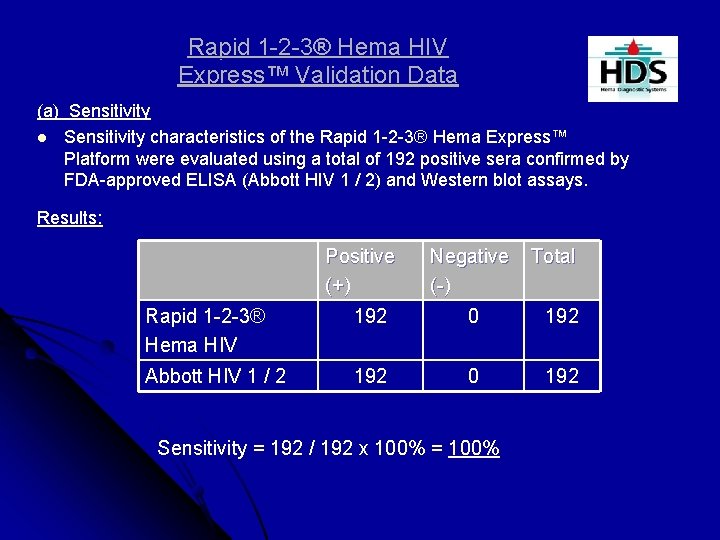
Rapid 1 -2 -3® Hema HIV Express™ Validation Data (a) Sensitivity l Sensitivity characteristics of the Rapid 1 -2 -3® Hema Express™ Platform were evaluated using a total of 192 positive sera confirmed by FDA-approved ELISA (Abbott HIV 1 / 2) and Western blot assays. Results: Positive (+) Negative (-) Total Rapid 1 -2 -3® Hema HIV 192 0 192 Abbott HIV 1 / 2 192 0 192 Sensitivity = 192 / 192 x 100% = 100%

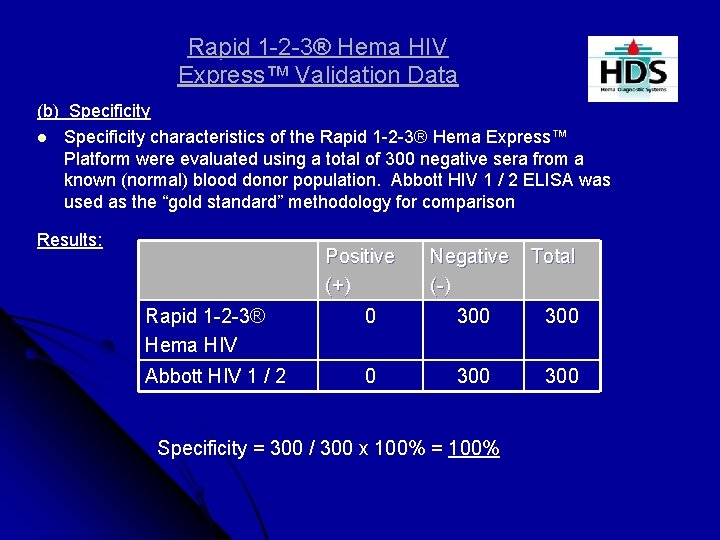
Rapid 1 -2 -3® Hema HIV Express™ Validation Data (b) Specificity l Specificity characteristics of the Rapid 1 -2 -3® Hema Express™ Platform were evaluated using a total of 300 negative sera from a known (normal) blood donor population. Abbott HIV 1 / 2 ELISA was used as the “gold standard” methodology for comparison Results: Positive (+) Negative (-) Total Rapid 1 -2 -3® Hema HIV 0 300 Abbott HIV 1 / 2 0 300 Specificity = 300 / 300 x 100% = 100%

SUMMARY OF ADDITIONAL AVAILABLE DATA l l l l Interfering Substances Low Titer Panels (BBI PRB 107) Anti HIV-1 Mixed Titer Panels (BBI PRB 202 / PRB 203) Anti HIV-2 Performance Panel (BBI PRF 202) Anti HIV 1 / 2 Combo Performance Panel (BBI PRZ 204) HIV-1 Seroconversion Panels (BBI PRB 959) HIV Seroconversion Panels (NABI SV 400 -403, SV 407 -408)

SUMMARY OF CLINICAL STUDIES AVAILABLE l l l l Clinical studies performed at University College Hospital, Ibadan, Nigeria Clinical studies performed at Mhu-Jhu Core Lab, New Mulago Hospital, Kampala, Uganda National Blood Transfusion Service, Kampala Uganda study Data presented at the 10 th Conference on Retroviruses and Opportunistic Infections, Boston USA 2003 National Institute of Communicable Diseases (NICD) study (Johannesburg, South Africa) Instituto Commemorativo Gorgas de Estudios de la Salud (GORGAS), Republic of Panama clinical study Laboratorio Nacional de Salud Publica, Santo Domingo, Dominican Republic clinical study

FEATURES / BENEFITS Rapid 1 -2 -3® Hema HIV EXPRESS™

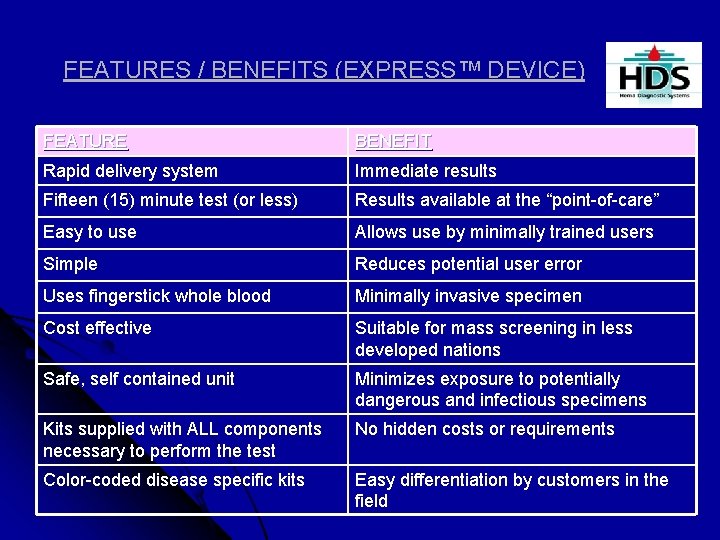
FEATURES / BENEFITS (EXPRESS™ DEVICE) FEATURE BENEFIT Rapid delivery system Immediate results Fifteen (15) minute test (or less) Results available at the “point-of-care” Easy to use Allows use by minimally trained users Simple Reduces potential user error Uses fingerstick whole blood Minimally invasive specimen Cost effective Suitable for mass screening in less developed nations Safe, self contained unit Minimizes exposure to potentially dangerous and infectious specimens Kits supplied with ALL components necessary to perform the test No hidden costs or requirements Color-coded disease specific kits Easy differentiation by customers in the field

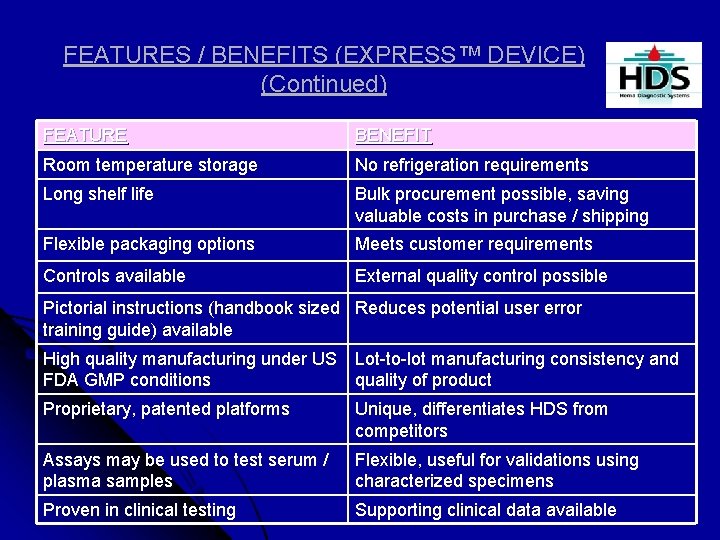
FEATURES / BENEFITS (EXPRESS™ DEVICE) (Continued) FEATURE BENEFIT Room temperature storage No refrigeration requirements Long shelf life Bulk procurement possible, saving valuable costs in purchase / shipping Flexible packaging options Meets customer requirements Controls available External quality control possible Pictorial instructions (handbook sized Reduces potential user error training guide) available High quality manufacturing under US Lot-to-lot manufacturing consistency and FDA GMP conditions quality of product Proprietary, patented platforms Unique, differentiates HDS from competitors Assays may be used to test serum / plasma samples Flexible, useful for validations using characterized specimens Proven in clinical testing Supporting clinical data available

What makes HDS and its products unique and different? l l l l Range of innovative, state-of-the-art assays/products supported by solid clinical data Flexible packaging to meet varying market conditions Simple/safe to administer, even by minimally trained personnel, in cases of staff shortage Excellent order process/fulfillment record Backed by outstanding support from well-trained staff Affordable without sacrificing performance Flexible financing for bulk volume purchases

CONCLUSIONS At HDS, we want to make a difference in the fight against HIV / AIDS, malaria, TB and other infectious diseases We listen to the needs of our Global customers, and build tools that can be used effectively in screening algorithms. We do this with the knowledge that resources (facilities, manpower, financing) is not always available. Consequently we develop high quality products at low cost

At HDS, we will not sacrifice quality or reliability of a product for price. Rather, we look for innovative ways to reduce costs and increase quality. Thank you for your hospitality in inviting me to speak to you today and thanks for listening!!

…DIAGNOSTIC SOLUTIONS FOR A CHANGING WORLDTM… info@rapid 123. com