Heat What is Temperature Quick write activity 5


















- Slides: 26

~Heat~

What is Temperature? Quick write activity: (5 minutes) What is temperature? How do you decide what temperature something is?

What is Temperature? Some things to think about: How hot is hot? How cold is cold? When we say something is hot or cold, is that a qualitative measure or a quantitative measure? When we say something is 50 degrees Celsius is that a qualitative measure or a quantitative measure?

Hot? Cold? Demo Three students will help me with this demonstration. Our procedure will be as follows. Step 1: Put one hand into the cold water. Put your other hand into the warm water. Step 2: Wait one minute. Take your hands out and put them in the room temperature water one hand at a time. Step 3: Each student describes the temperatures they felt. Can you rely on your hands to determine temperature? Why or why not?

How thermometers work Liquid thermometers work because of thermal expansion. Thermal expansion is the increase of volume of a substance due to an increase in temperature. Many thermometers use either mercury or alcohol because they remain in the liquid form over a large range of temperatures.

Kinetic Energy Temperature is the measure of the average kinetic energy of particles. Kinetic energy is another way to talk about the amount of movement particles are doing. Higher temperature = more kinetic energy

How thermometers work Imagine this: A dry sponge is our imaginary liquid. The water you add to the sponge would be the heat. When the water interacts with the dry sponge, the sponge expands. The amount of sponge doesn’t change, but it spreads apart as it increases in temperature (from more heat).

How thermometers work Thermometers are often made of glass with a tiny little glass tube in the center for the liquid to travel. Thermal expansion isn’t much for the liquid inside thermometer, so the size of the inner tube must be VERY small in order to show us the change in temperature

What is heat? • Heat is the transfer of energy between objects that are at different temperatures – Heat energy is ALWAYS transferred from the object with higher temperature to the object with lower temperature.

Absolute Zero Water freezes at 0 degrees Celsius. 273 Kelvin equals 0 degrees Celsius. Absolute ZERO would be -273 Celsius.

What is thermal energy? • If heat is the transfer of energy, then thermal energy is the type of energy being transferred between objects. • Thermal energy can move between objects in three different ways. – These are conduction, convection, and radiation.

Conduction • Conduction is simply the transfer of thermal energy through direct contact. Conduction can also occur within a substance

Convection • Convection is the transfer of thermal energy by movement of a liquid or gas. The liquid or gas cycles much like a turning bicycle wheel. (Think: Toaster Oven/Oven)

Radiation • Radiation is the transfer of thermal energy through matter or space by electromagnetic waves, visible light, or infrared waves. ALL objects emit radiation. (Think: Sunbathing)

Back to Conduction! Do these items look familiar?

What do they have in common? All the items in the previous slide are insulators. An insulator is a substance that does not conduct thermal energy well and reduces the amount of heat transfer. Substances that DO conduct thermal energy well are called conductors.

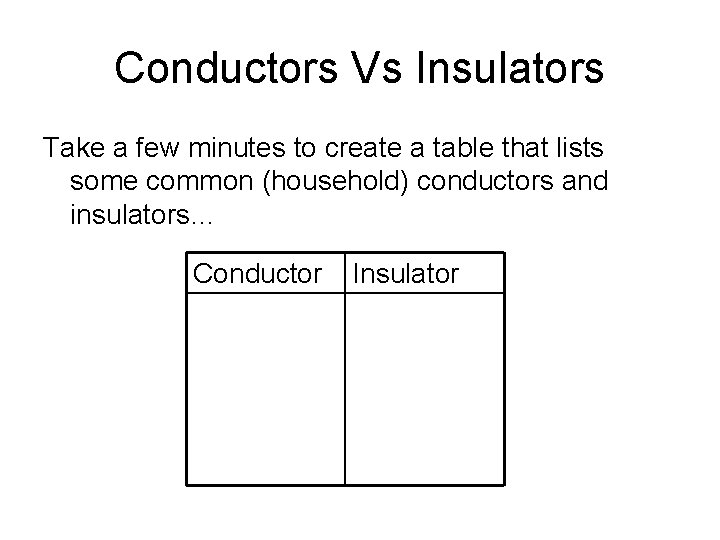
Conductors Vs Insulators Take a few minutes to create a table that lists some common (household) conductors and insulators… Conductor Insulator

Phases of Matter There are 3 phases of matter -solids: particles vibrate in place -liquids: particles slide past each other -gases: particles move independently of each other Moving from one phase to another is called changing states of matter. Some examples of changing states are freezing, boiling, condensing, and melting.

Pre-Lab for next class Before we can do our lab on thermal energy, temperature, and heat next class we need to prepare ourselves. This should be written into your science journal as “Heat Lab”

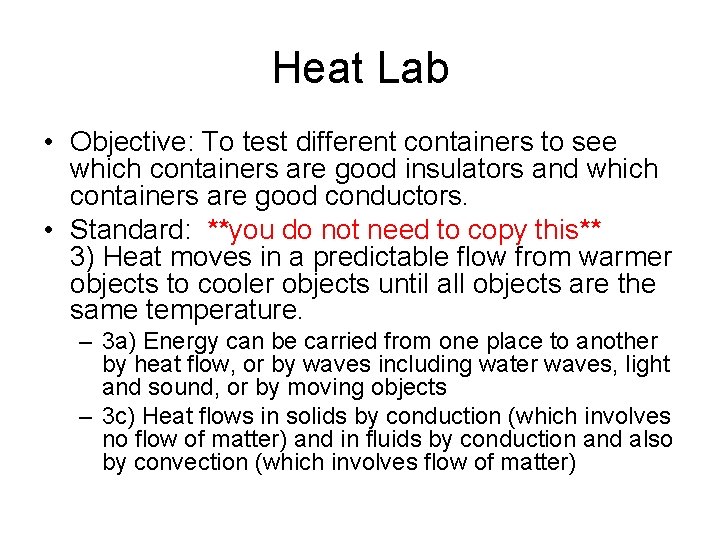
Heat Lab • Objective: To test different containers to see which containers are good insulators and which containers are good conductors. • Standard: **you do not need to copy this** 3) Heat moves in a predictable flow from warmer objects to cooler objects until all objects are the same temperature. – 3 a) Energy can be carried from one place to another by heat flow, or by waves including water waves, light and sound, or by moving objects – 3 c) Heat flows in solids by conduction (which involves no flow of matter) and in fluids by conduction and also by convection (which involves flow of matter)

Heat Lab Please take 5 minutes to create a hypothesis for the lab. You will have one of the following containers: Styrofoam, metal, or glass. Your hypothesis should include which of these you think will be the best conductor and which will be the best insulator and why you believe that. Hypothesis: I believe… (or I think…)**you write this part, leave yourself a few lines**

Heat Lab Procedure: Step 1) Obtain materials: large container, one smaller container type, stopwatch or timer, 2 thermometers, ice water, warm water. Step 2) Place the warm water in the small container and the cold water in the big container. Place a thermometer in each.

Heat Lab Step 3) Take initial readings of the ice water and warm water SEPERATELY for 2 -3 minutes every 30 seconds (4 -6 readings) Do not let thermometer rest against the container. Step 4) Place the small container in the big container and record temperature readings for both the big and small containers every 30 seconds for 10 minutes.

Heat Lab Step 5) Continue to take temperature readings every minute (instead of 30 seconds) for the next 10 minutes.

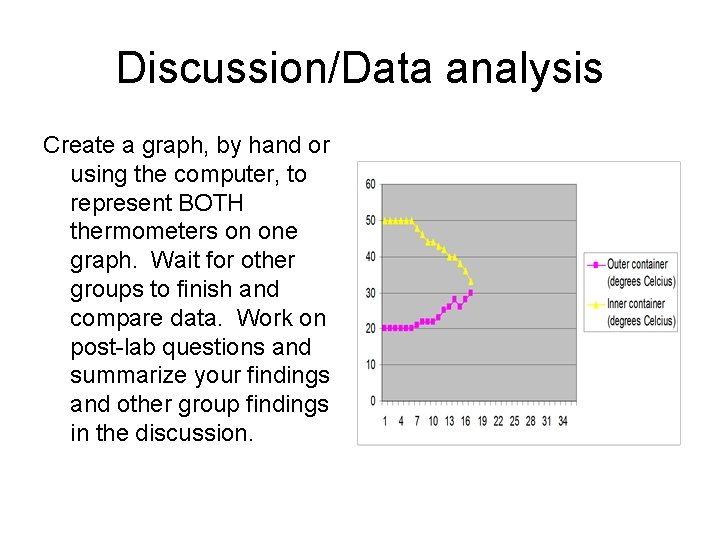
Discussion/Data analysis Create a graph, by hand or using the computer, to represent BOTH thermometers on one graph. Wait for other groups to finish and compare data. Work on post-lab questions and summarize your findings and other group findings in the discussion.

Conclusion Write a conclusion that reflects your understanding of the entire lab and discuss your results. How did they compare to your hypothesis? The conclusion should be a half-page. IT WILL BE IN THE NOTEBOOK CHECK