Heat Treatment Microstructure and Properties of Carbon Steels
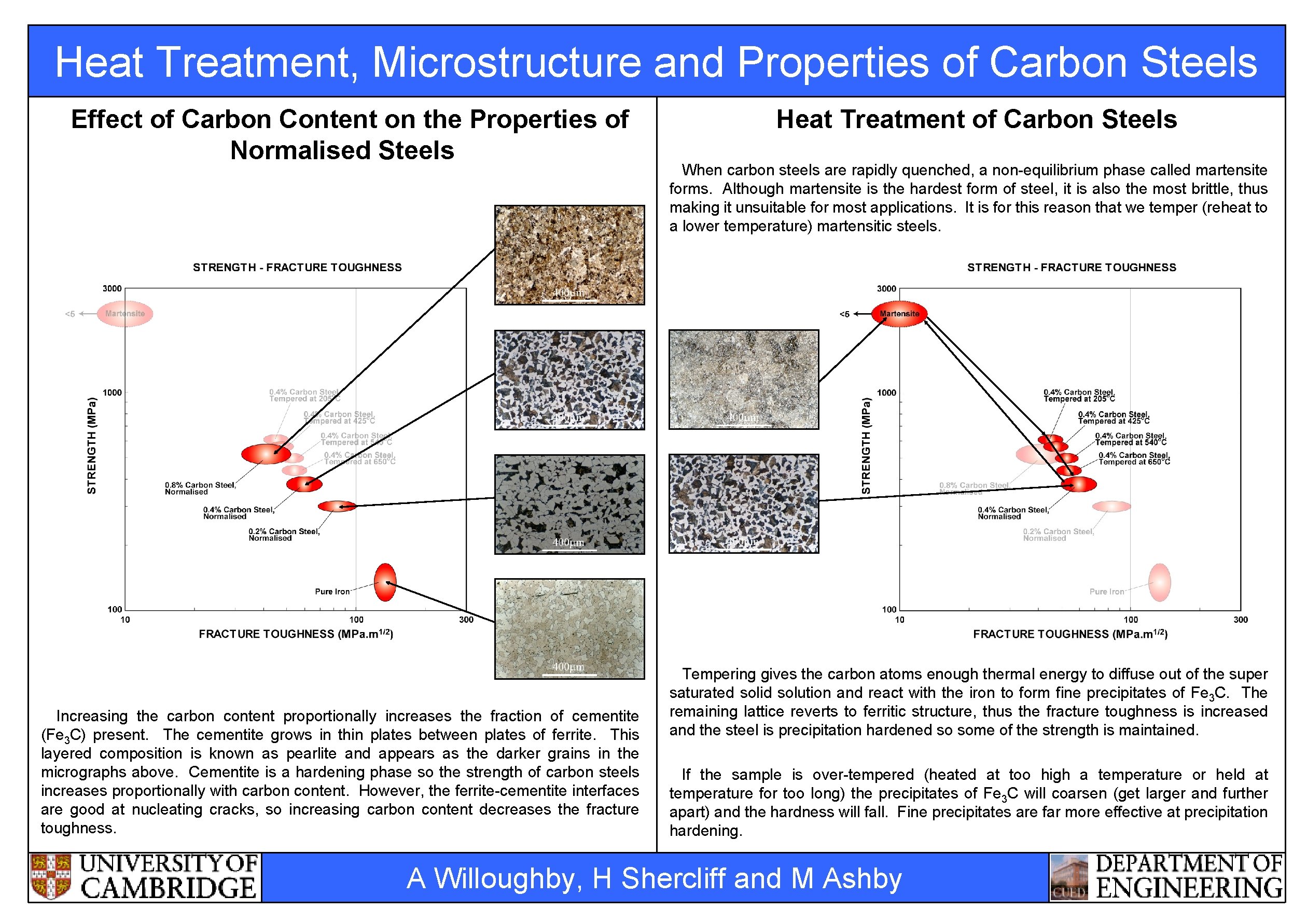
Heat Treatment, Microstructure and Properties of Carbon Steels Effect of Carbon Content on the Properties of Normalised Steels Increasing the carbon content proportionally increases the fraction of cementite (Fe 3 C) present. The cementite grows in thin plates between plates of ferrite. This layered composition is known as pearlite and appears as the darker grains in the micrographs above. Cementite is a hardening phase so the strength of carbon steels increases proportionally with carbon content. However, the ferrite-cementite interfaces are good at nucleating cracks, so increasing carbon content decreases the fracture toughness. Heat Treatment of Carbon Steels When carbon steels are rapidly quenched, a non-equilibrium phase called martensite forms. Although martensite is the hardest form of steel, it is also the most brittle, thus making it unsuitable for most applications. It is for this reason that we temper (reheat to a lower temperature) martensitic steels. Tempering gives the carbon atoms enough thermal energy to diffuse out of the super saturated solid solution and react with the iron to form fine precipitates of Fe 3 C. The remaining lattice reverts to ferritic structure, thus the fracture toughness is increased and the steel is precipitation hardened so some of the strength is maintained. If the sample is over-tempered (heated at too high a temperature or held at temperature for too long) the precipitates of Fe 3 C will coarsen (get larger and further apart) and the hardness will fall. Fine precipitates are far more effective at precipitation hardening. A Willoughby, H Shercliff and M Ashby
- Slides: 1