Heat Transfer Problems Calorimeter an insulated container that
Heat Transfer Problems
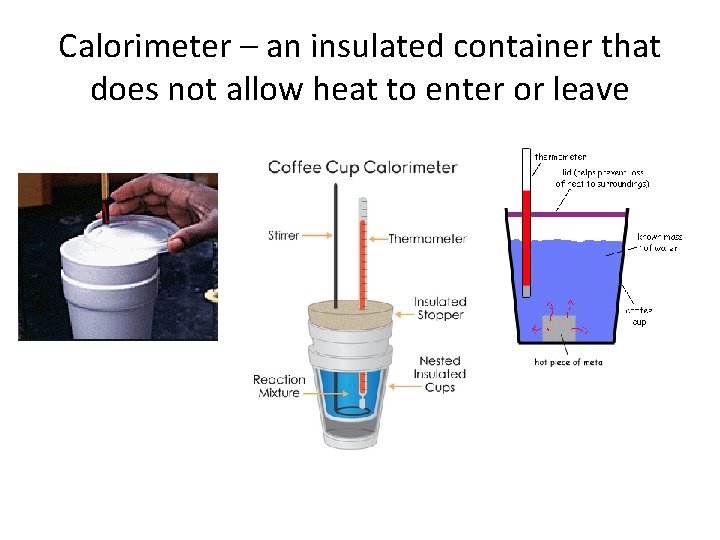
Calorimeter – an insulated container that does not allow heat to enter or leave
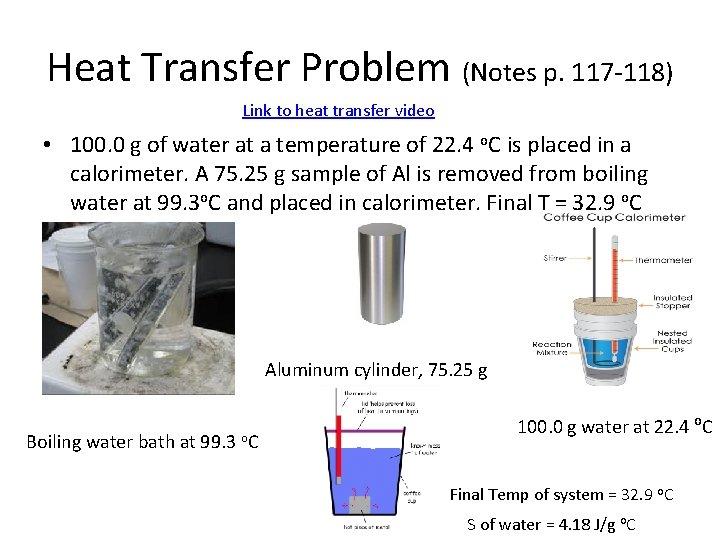
Heat Transfer Problem (Notes p. 117 -118) Link to heat transfer video • 100. 0 g of water at a temperature of 22. 4 o. C is placed in a calorimeter. A 75. 25 g sample of Al is removed from boiling water at 99. 3 o. C and placed in calorimeter. Final T = 32. 9 o. C Aluminum cylinder, 75. 25 g Boiling water bath at 99. 3 o. C 100. 0 g water at 22. 4 o. C Final Temp of system = 32. 9 o. C S of water = 4. 18 J/g o. C
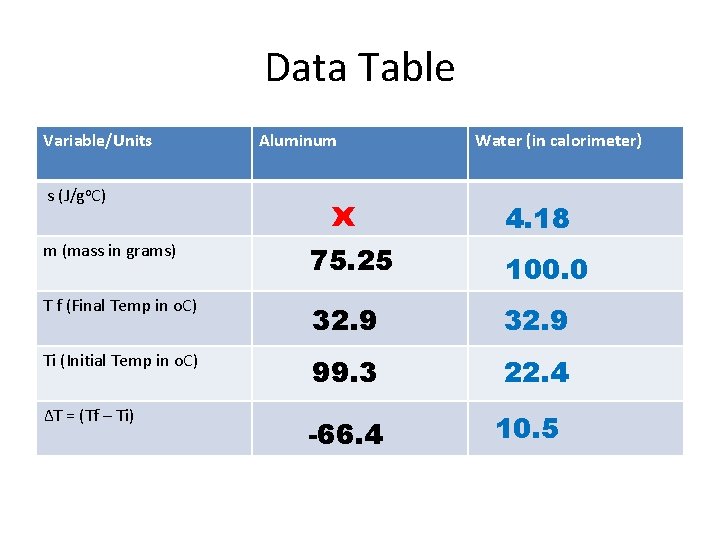
Data Table Variable/Units s (J/go. C) Aluminum x Water (in calorimeter) 4. 18 m (mass in grams) 75. 25 100. 0 T f (Final Temp in o. C) 32. 9 Ti (Initial Temp in o. C) 99. 3 22. 4 ∆T = (Tf – Ti) -66. 4 10. 5
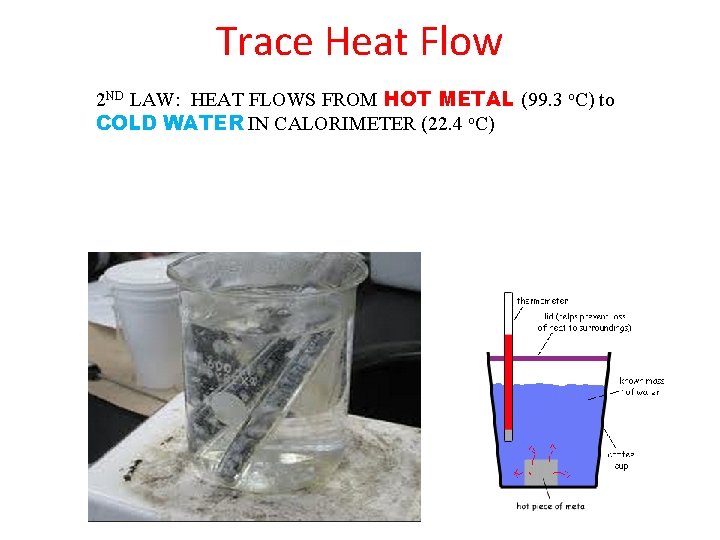
Trace Heat Flow 2 ND LAW: HEAT FLOWS FROM HOT METAL (99. 3 o. C) to COLD WATER IN CALORIMETER (22. 4 o. C)
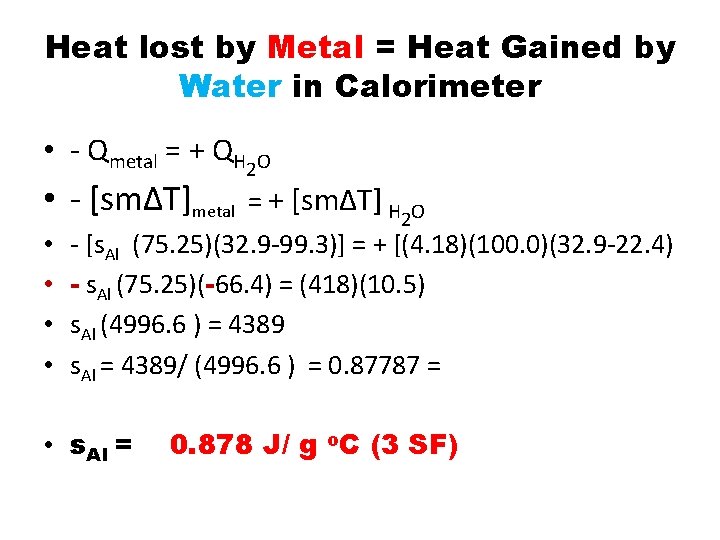
Heat lost by Metal = Heat Gained by Water in Calorimeter • - Qmetal = + QH 2 O • - [sm∆T]metal = + [sm∆T] H 2 O • • - [s. Al (75. 25)(32. 9 -99. 3)] = + [(4. 18)(100. 0)(32. 9 -22. 4) - s. Al (75. 25)(-66. 4) = (418)(10. 5) s. Al (4996. 6 ) = 4389 s. Al = 4389/ (4996. 6 ) = 0. 87787 = • s. Al = 0. 878 J/ g o. C (3 SF)
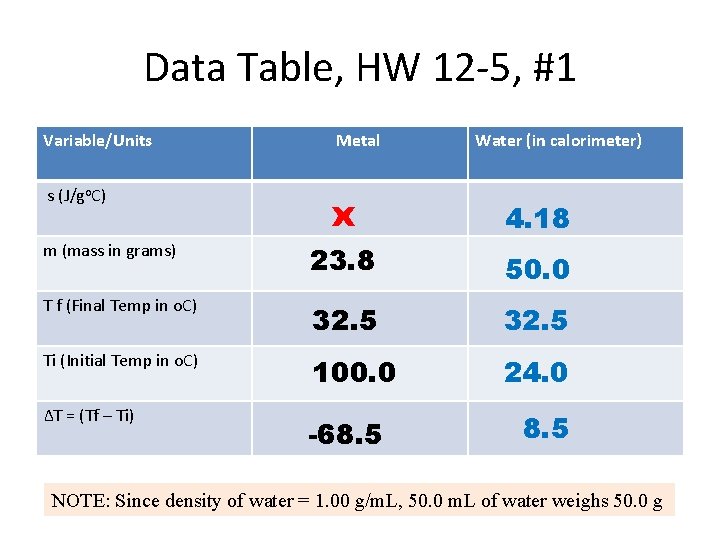
Data Table, HW 12 -5, #1 Variable/Units Metal Water (in calorimeter) s (J/go. C) x 4. 18 m (mass in grams) 23. 8 50. 0 T f (Final Temp in o. C) 32. 5 Ti (Initial Temp in o. C) 100. 0 24. 0 ∆T = (Tf – Ti) -68. 5 NOTE: Since density of water = 1. 00 g/m. L, 50. 0 m. L of water weighs 50. 0 g
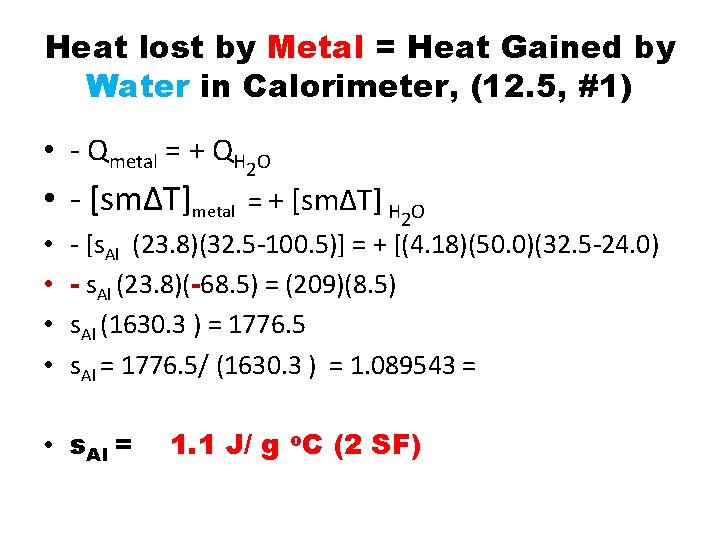
Heat lost by Metal = Heat Gained by Water in Calorimeter, (12. 5, #1) • - Qmetal = + QH 2 O • - [sm∆T]metal = + [sm∆T] H 2 O • • - [s. Al (23. 8)(32. 5 -100. 5)] = + [(4. 18)(50. 0)(32. 5 -24. 0) - s. Al (23. 8)(-68. 5) = (209)(8. 5) s. Al (1630. 3 ) = 1776. 5 s. Al = 1776. 5/ (1630. 3 ) = 1. 089543 = • s. Al = 1. 1 J/ g o. C (2 SF)
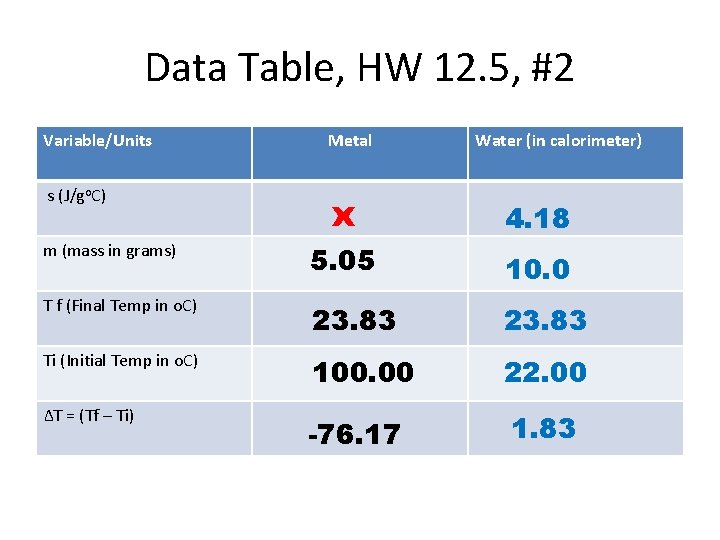
Data Table, HW 12. 5, #2 Variable/Units Metal Water (in calorimeter) s (J/go. C) x 4. 18 m (mass in grams) 5. 05 10. 0 T f (Final Temp in o. C) 23. 83 Ti (Initial Temp in o. C) 100. 00 22. 00 ∆T = (Tf – Ti) -76. 17 1. 83
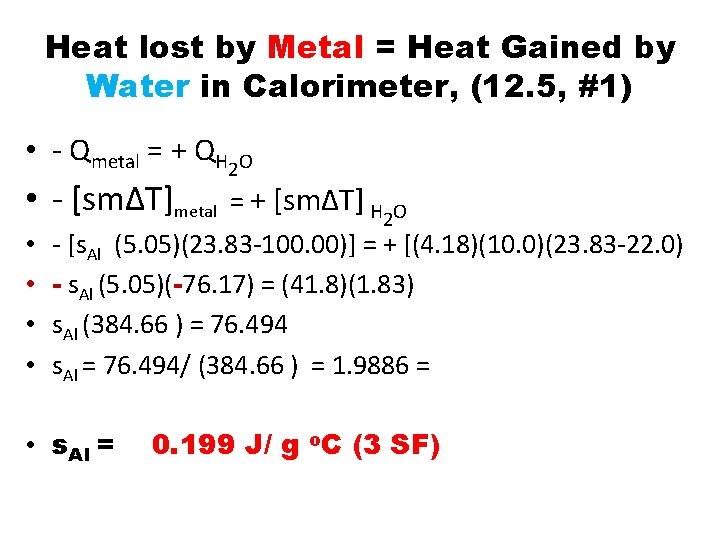
Heat lost by Metal = Heat Gained by Water in Calorimeter, (12. 5, #1) • - Qmetal = + QH 2 O • - [sm∆T]metal = + [sm∆T] H 2 O • • - [s. Al (5. 05)(23. 83 -100. 00)] = + [(4. 18)(10. 0)(23. 83 -22. 0) - s. Al (5. 05)(-76. 17) = (41. 8)(1. 83) s. Al (384. 66 ) = 76. 494 s. Al = 76. 494/ (384. 66 ) = 1. 9886 = • s. Al = 0. 199 J/ g o. C (3 SF)
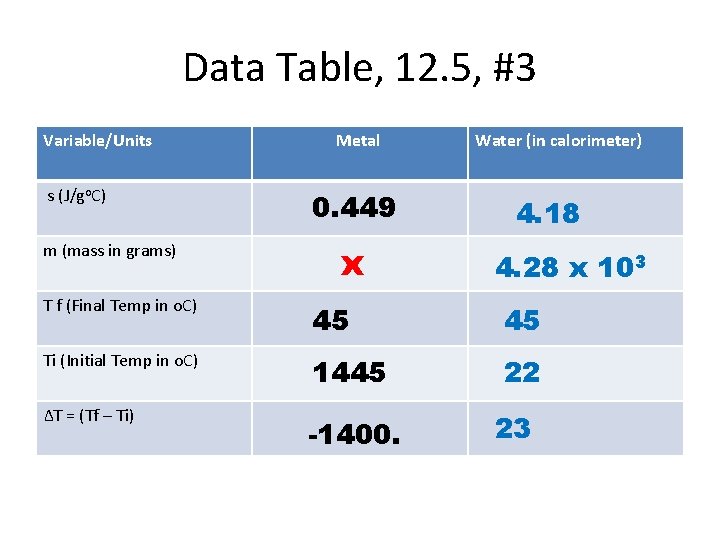
Data Table, 12. 5, #3 Variable/Units s (J/go. C) m (mass in grams) Metal 0. 449 x Water (in calorimeter) 4. 18 4. 28 x 103 T f (Final Temp in o. C) 45 45 Ti (Initial Temp in o. C) 1445 22 ∆T = (Tf – Ti) -1400. 23
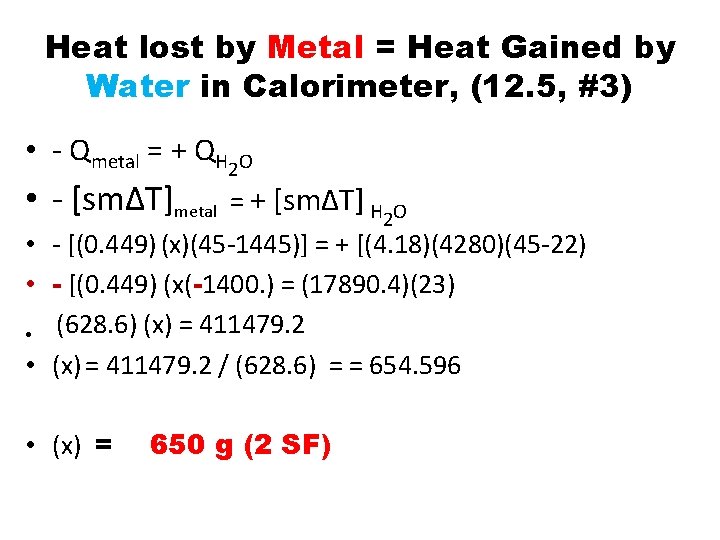
Heat lost by Metal = Heat Gained by Water in Calorimeter, (12. 5, #3) • - Qmetal = + QH 2 O • - [sm∆T]metal = + [sm∆T] H 2 O • - [(0. 449) (x)(45 -1445)] = + [(4. 18)(4280)(45 -22) • - [(0. 449) (x(-1400. ) = (17890. 4)(23) • (628. 6) (x) = 411479. 2 • (x) = 411479. 2 / (628. 6) = = 654. 596 • (x) = 650 g (2 SF)
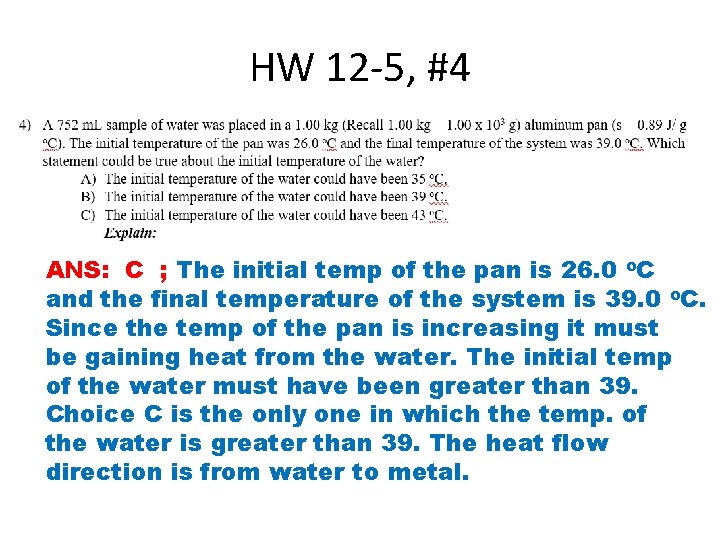
HW 12 -5, #4 ANS: C ; The initial temp of the pan is 26. 0 o. C and the final temperature of the system is 39. 0 o. C. Since the temp of the pan is increasing it must be gaining heat from the water. The initial temp of the water must have been greater than 39. Choice C is the only one in which the temp. of the water is greater than 39. The heat flow direction is from water to metal.

HW 12 -5, #5 ANS = C. The iron and the water have equal masses, the only difference is the specific heat capacity terms (0. 449 vs 4. 18). Since the s for water is almost a factor 10 greater than s for iron the temp of water will rise much less when it absorbs the heat lost by the iron. Q = sm∆T ; ∆T = Q/ sm; Iron: ∆T = Q/(0. 449)(100) Water: ∆T = Q/(4. 18)(100) Q’s are =, m are =; Iron ∆T will ≈ factor of 10 greater Iron ∆T = 1/0. 449 = 2. 22 ; Water ∆T = 1/4. 18 = 0. 23

HW 12 -5, #6 ANS: B ; Since the two substances are both identical (water) the s values are identical and the masses are exactly equal the TF will exactly halfway between the Ti of each.
- Slides: 15