Heat Transfer Mechanisms Dr AA Department of Chemical






- Slides: 41

Heat Transfer Mechanisms Dr. AA Department of Chemical Engineering University Teknology Malaysia

Conduction Dr. AA Department of Chemical Engineering University Teknology Malaysia

Heat Conduction Key Question: How does heat pass through different materials?

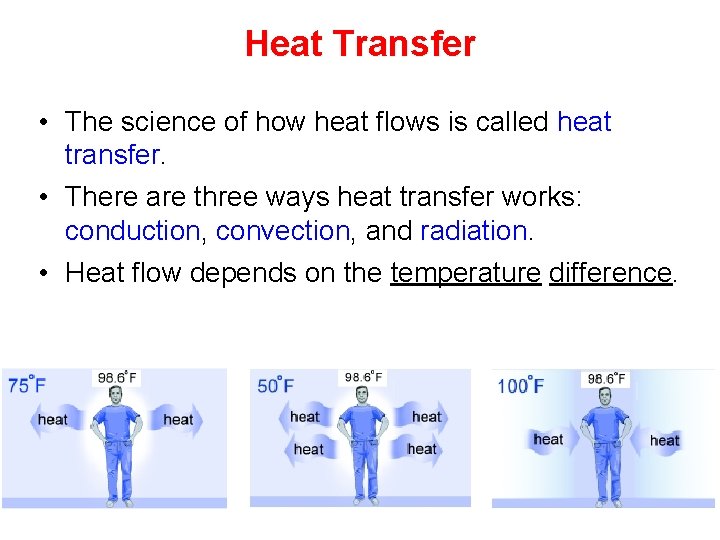
Heat Transfer • The science of how heat flows is called heat transfer. • There are three ways heat transfer works: conduction, convection, and radiation. • Heat flow depends on the temperature difference.

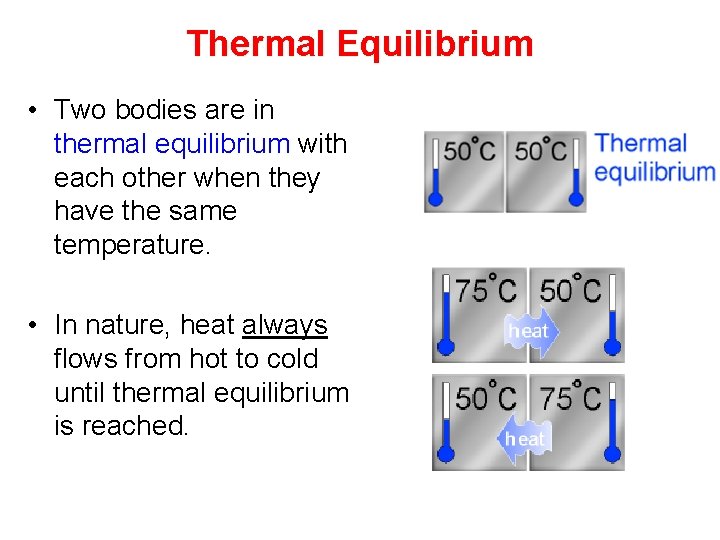
Thermal Equilibrium • Two bodies are in thermal equilibrium with each other when they have the same temperature. • In nature, heat always flows from hot to cold until thermal equilibrium is reached.

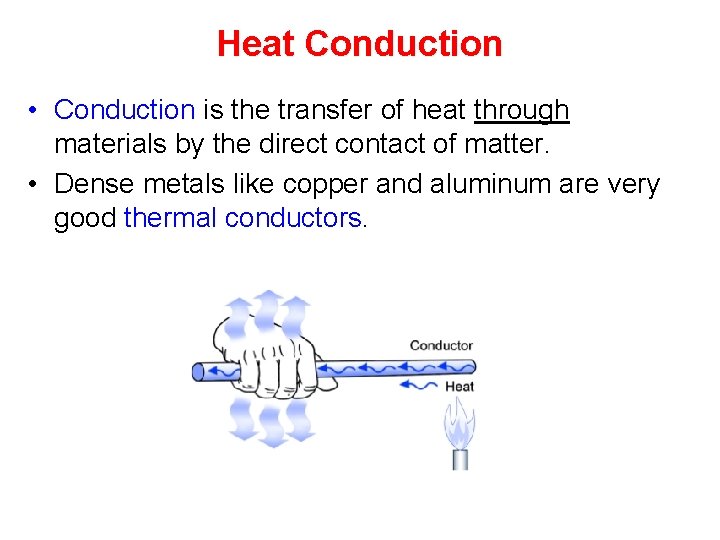
Heat Conduction • Conduction is the transfer of heat through materials by the direct contact of matter. • Dense metals like copper and aluminum are very good thermal conductors.

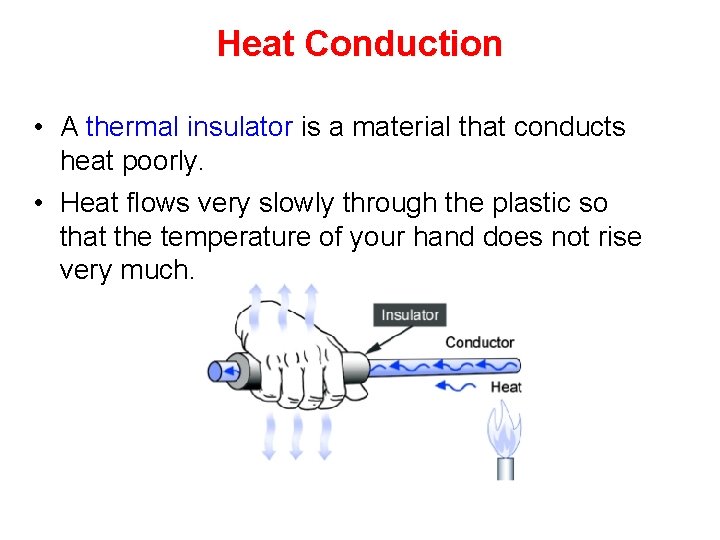
Heat Conduction • A thermal insulator is a material that conducts heat poorly. • Heat flows very slowly through the plastic so that the temperature of your hand does not rise very much.

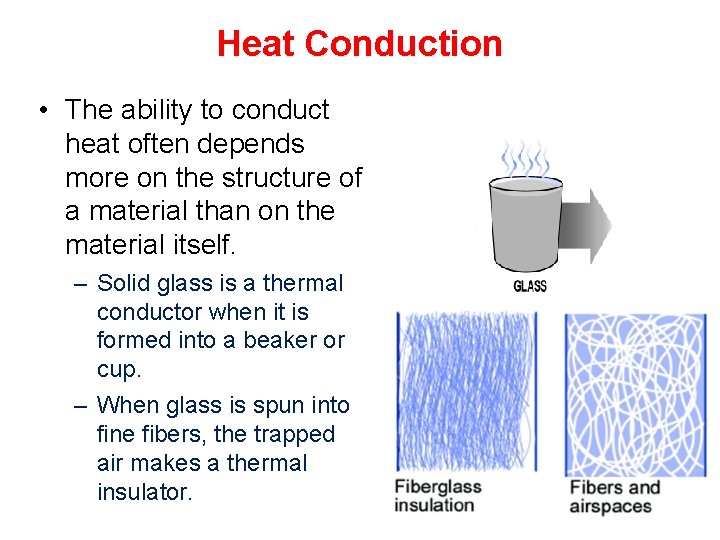
Heat Conduction • The ability to conduct heat often depends more on the structure of a material than on the material itself. – Solid glass is a thermal conductor when it is formed into a beaker or cup. – When glass is spun into fine fibers, the trapped air makes a thermal insulator.

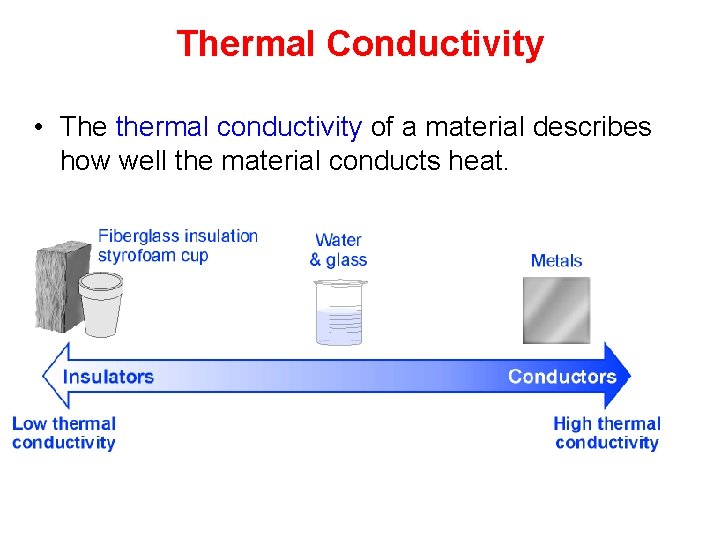
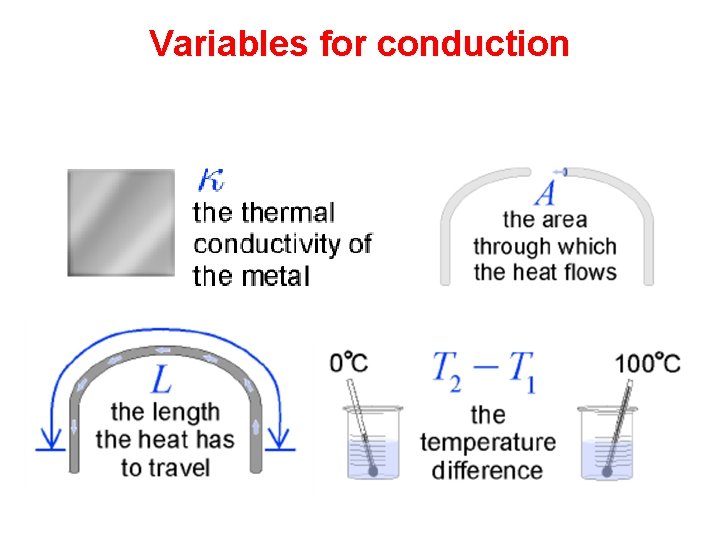
Thermal Conductivity • The thermal conductivity of a material describes how well the material conducts heat.

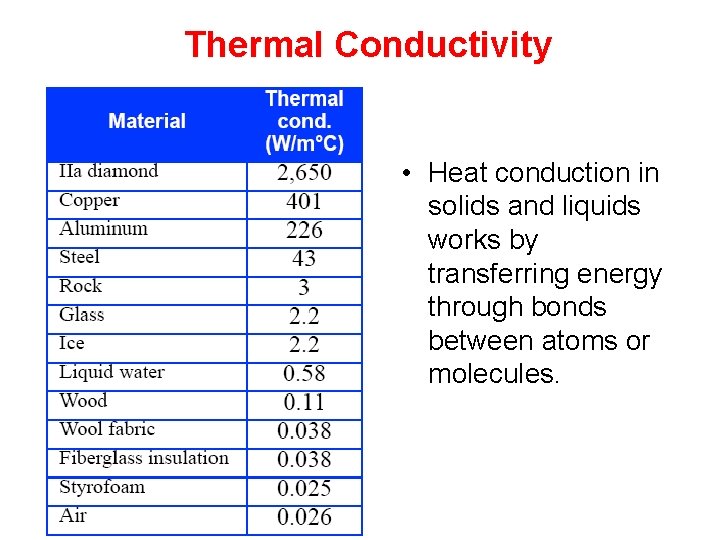
Thermal Conductivity • Heat conduction in solids and liquids works by transferring energy through bonds between atoms or molecules.

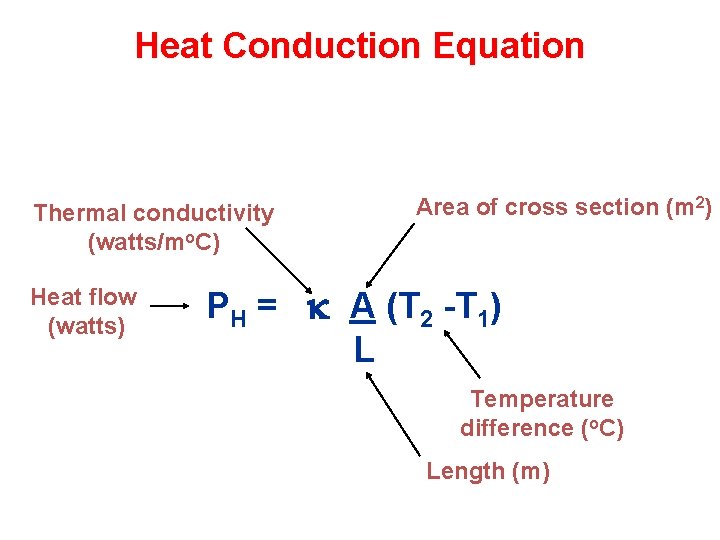
Heat Conduction Equation Thermal conductivity (watts/mo. C) Heat flow (watts) Area of cross section (m 2) PH = k A (T 2 -T 1) L Temperature difference (o. C) Length (m)

Variables for conduction

Convection Dr. AA Department of Chemical Engineering University Teknology Malaysia

Convection Key Question: Can moving matter carry thermal energy?

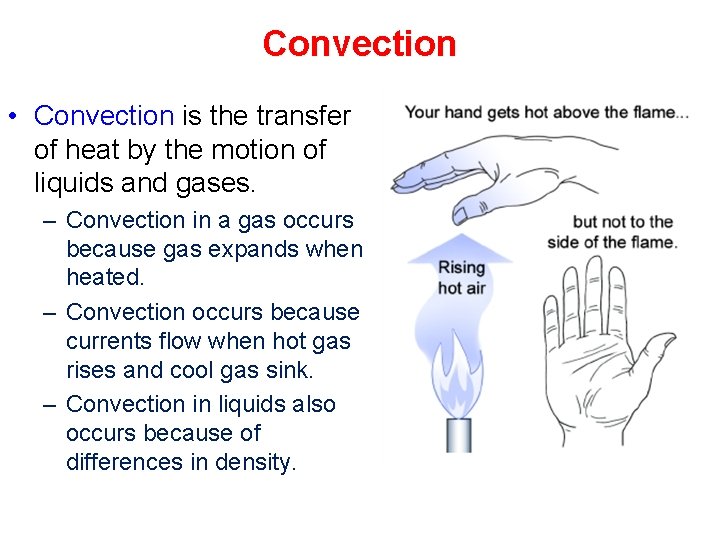
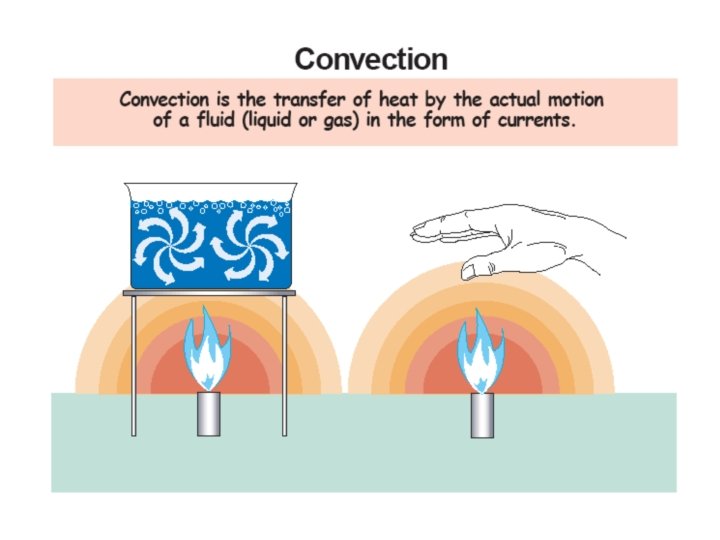
Convection • Convection is the transfer of heat by the motion of liquids and gases. – Convection in a gas occurs because gas expands when heated. – Convection occurs because currents flow when hot gas rises and cool gas sink. – Convection in liquids also occurs because of differences in density.


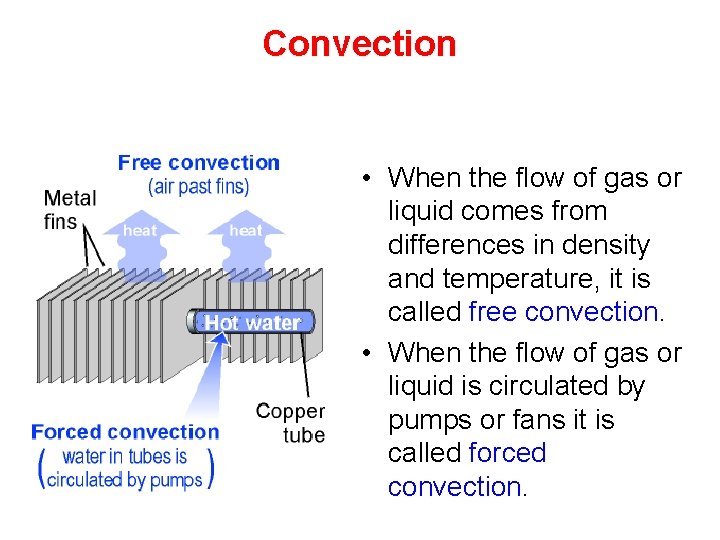
Convection • When the flow of gas or liquid comes from differences in density and temperature, it is called free convection. • When the flow of gas or liquid is circulated by pumps or fans it is called forced convection.

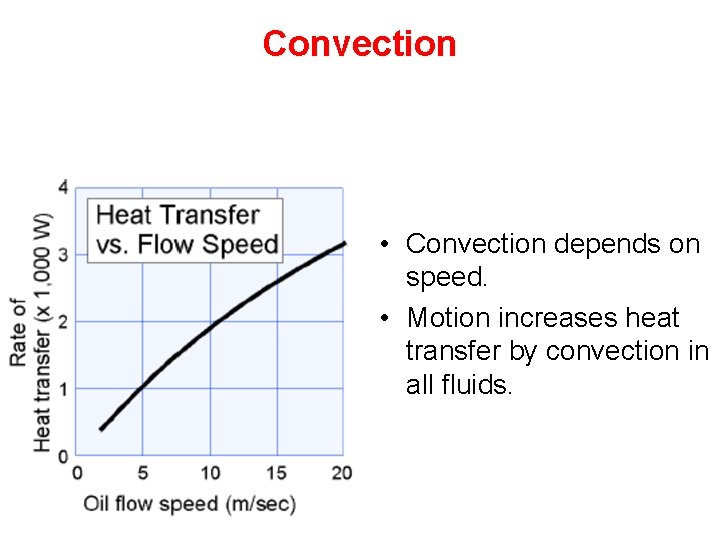
Convection • Convection depends on speed. • Motion increases heat transfer by convection in all fluids.

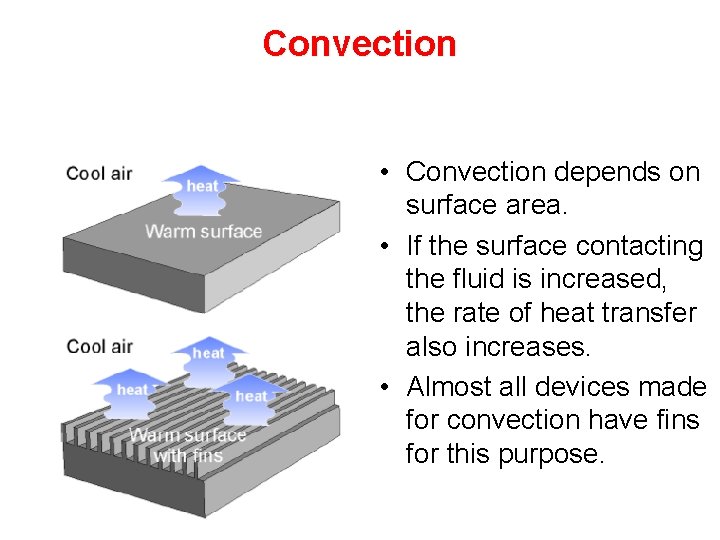
Convection • Convection depends on surface area. • If the surface contacting the fluid is increased, the rate of heat transfer also increases. • Almost all devices made for convection have fins for this purpose.

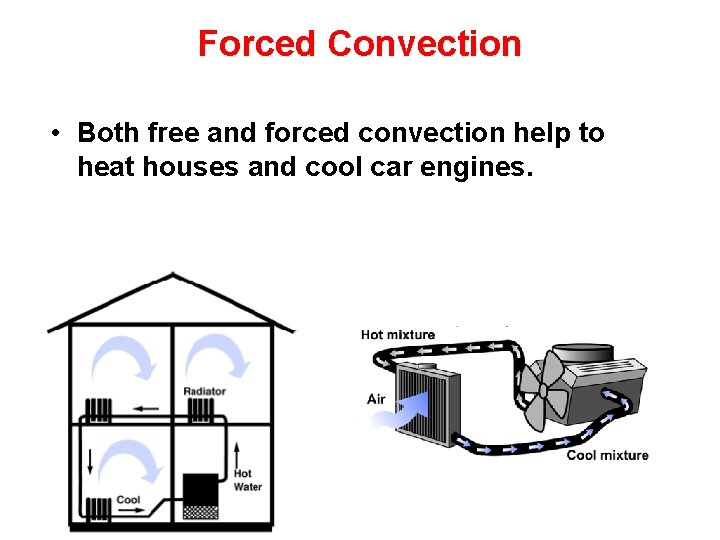
Forced Convection • Both free and forced convection help to heat houses and cool car engines.

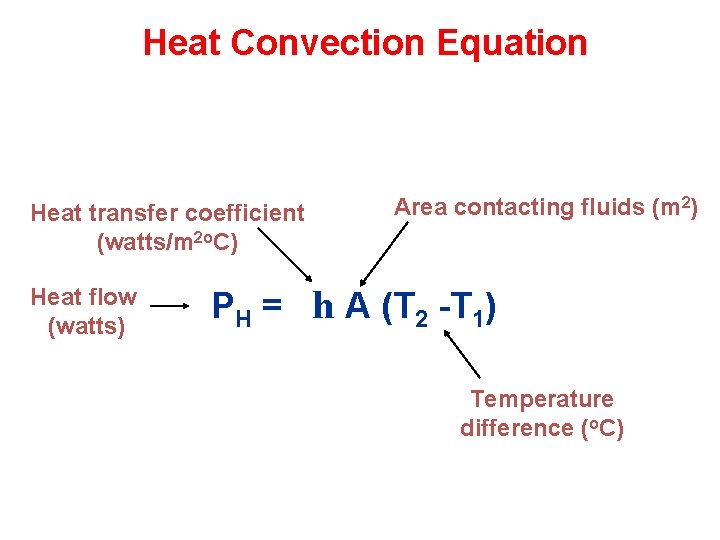
Heat Convection Equation Heat transfer coefficient (watts/m 2 o. C) Heat flow (watts) Area contacting fluids (m 2) PH = h A (T 2 -T 1) Temperature difference (o. C)

Radiation Dr. AA Department of Chemical Engineering University Teknology Malaysia


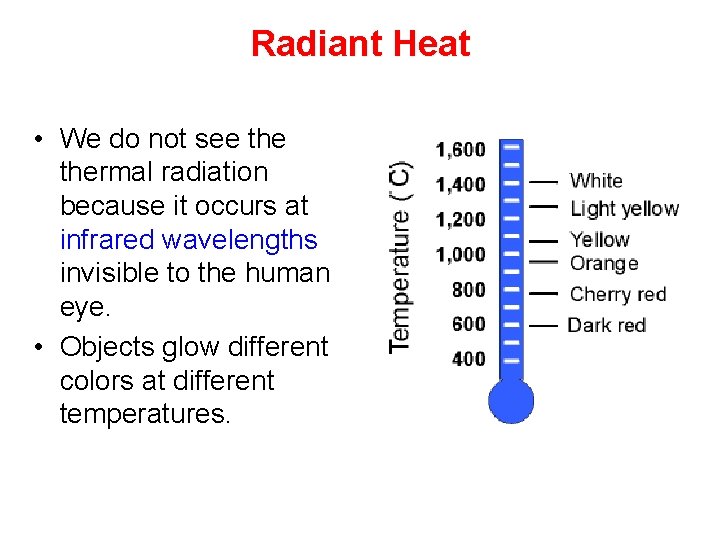
Radiant Heat • We do not see thermal radiation because it occurs at infrared wavelengths invisible to the human eye. • Objects glow different colors at different temperatures.

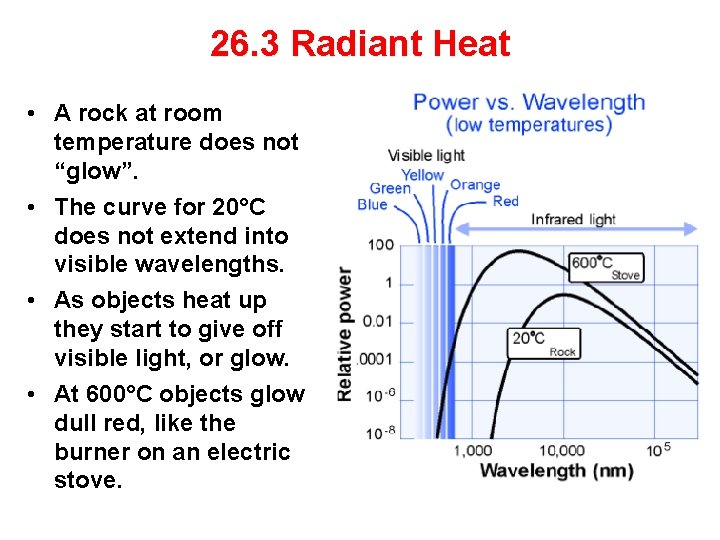
26. 3 Radiant Heat • A rock at room temperature does not “glow”. • The curve for 20°C does not extend into visible wavelengths. • As objects heat up they start to give off visible light, or glow. • At 600°C objects glow dull red, like the burner on an electric stove.

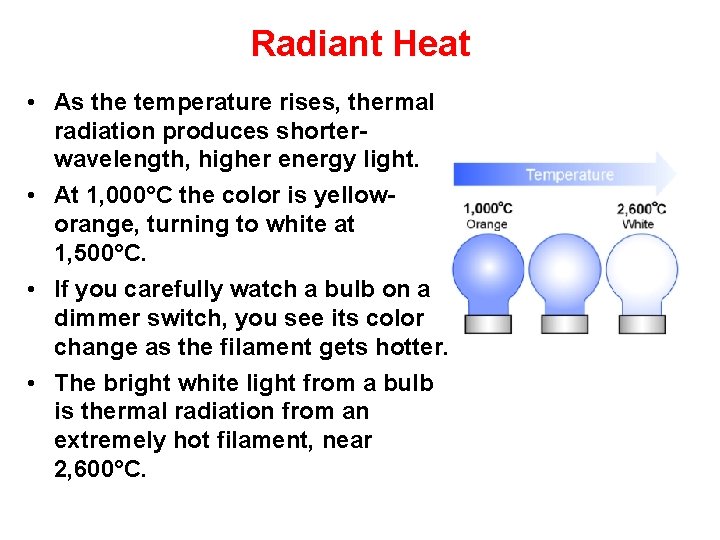
Radiant Heat • As the temperature rises, thermal radiation produces shorterwavelength, higher energy light. • At 1, 000°C the color is yelloworange, turning to white at 1, 500°C. • If you carefully watch a bulb on a dimmer switch, you see its color change as the filament gets hotter. • The bright white light from a bulb is thermal radiation from an extremely hot filament, near 2, 600°C.

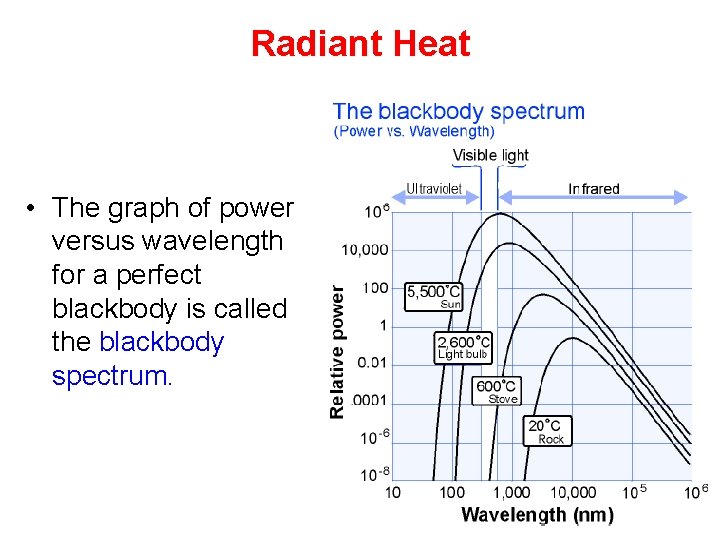
Radiant Heat • The graph of power versus wavelength for a perfect blackbody is called the blackbody spectrum.

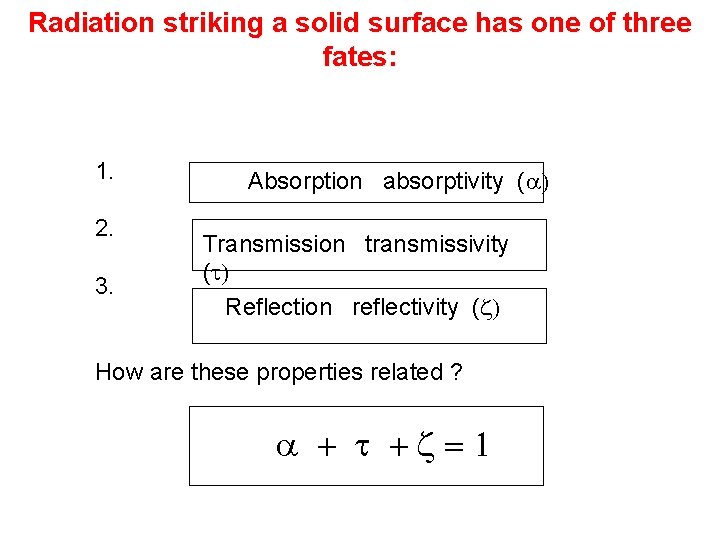
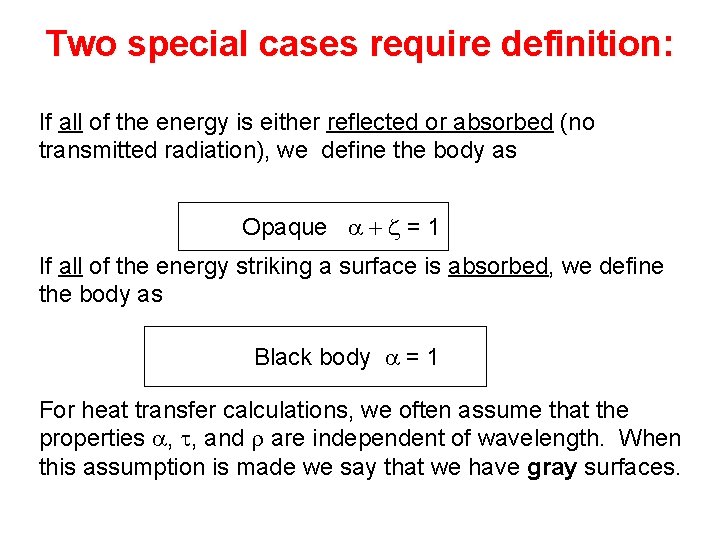
Radiation striking a solid surface has one of three fates: 1. 2. 3. Absorption absorptivity (a) Transmission transmissivity (t) Reflection reflectivity (z) How are these properties related ? a + t +z=1

Two special cases require definition: If all of the energy is either reflected or absorbed (no transmitted radiation), we define the body as Opaque a + z = 1 If all of the energy striking a surface is absorbed, we define the body as Black body a = 1 For heat transfer calculations, we often assume that the properties a, t, and r are independent of wavelength. When this assumption is made we say that we have gray surfaces.

Let us return to the subject of radiation emitted by a surface. Total emissive power is defined as the total amount of energy leaving the surface per unit time per unit area: W = energy/area-time [Btu/hr-ft 2 or W/m 2] Note: Emissive power is a function of wavelength. The important wavelengths for heat transfer are 0. 5 -50 µm. For temperatures above 1500°F, the important wavelength range is between 0. 5 and 5 µm. In our analysis, we will use the average values over all wavelengths.

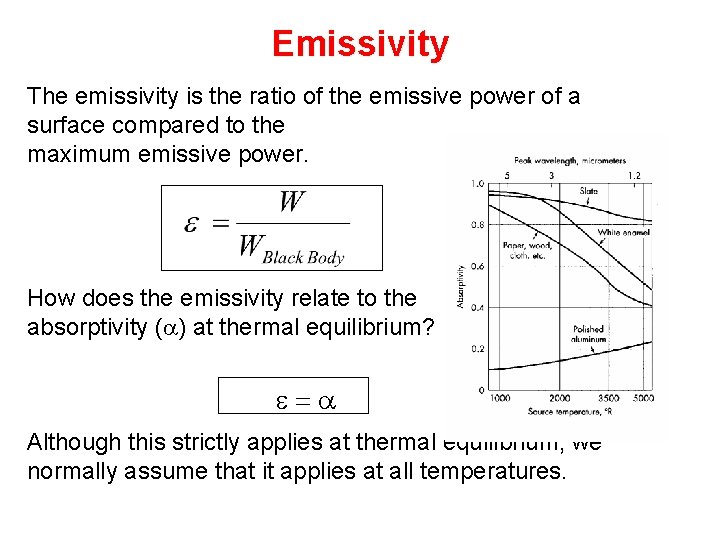
Emissivity The emissivity is the ratio of the emissive power of a surface compared to the maximum emissive power. How does the emissivity relate to the absorptivity (a) at thermal equilibrium? e=a Although this strictly applies at thermal equilibrium, we normally assume that it applies at all temperatures.

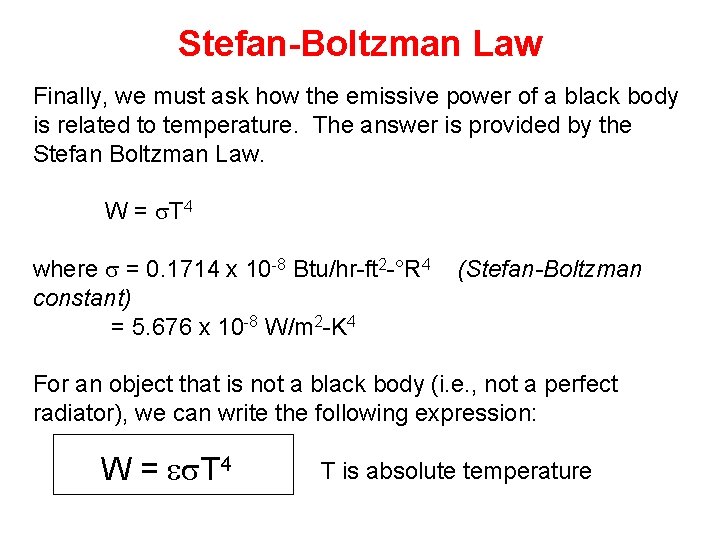
Stefan-Boltzman Law Finally, we must ask how the emissive power of a black body is related to temperature. The answer is provided by the Stefan Boltzman Law. W = s. T 4 where s = 0. 1714 x 10 -8 Btu/hr-ft 2 -°R 4 constant) = 5. 676 x 10 -8 W/m 2 -K 4 (Stefan-Boltzman For an object that is not a black body (i. e. , not a perfect radiator), we can write the following expression: W = es. T 4 T is absolute temperature

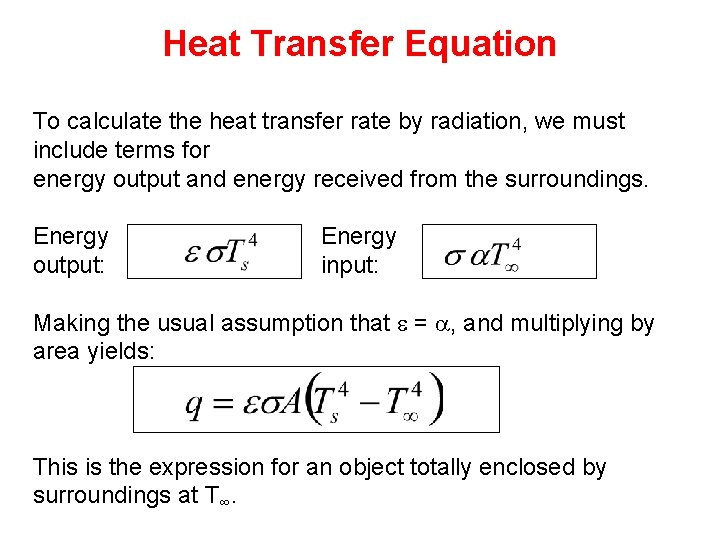
Heat Transfer Equation To calculate the heat transfer rate by radiation, we must include terms for energy output and energy received from the surroundings. Energy output: Energy input: Making the usual assumption that e = a, and multiplying by area yields: This is the expression for an object totally enclosed by surroundings at T∞.

View factors

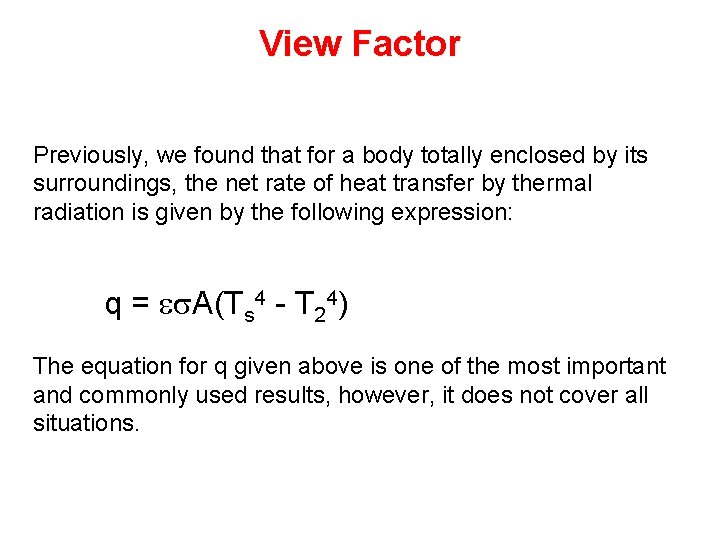
View Factor Previously, we found that for a body totally enclosed by its surroundings, the net rate of heat transfer by thermal radiation is given by the following expression: q = es. A(Ts 4 - T 24) The equation for q given above is one of the most important and commonly used results, however, it does not cover all situations.

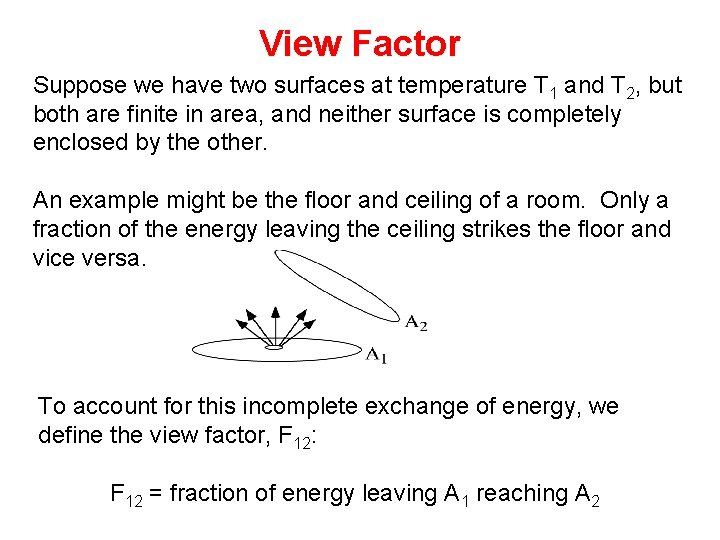
View Factor Suppose we have two surfaces at temperature T 1 and T 2, but both are finite in area, and neither surface is completely enclosed by the other. An example might be the floor and ceiling of a room. Only a fraction of the energy leaving the ceiling strikes the floor and vice versa. To account for this incomplete exchange of energy, we define the view factor, F 12: F 12 = fraction of energy leaving A 1 reaching A 2

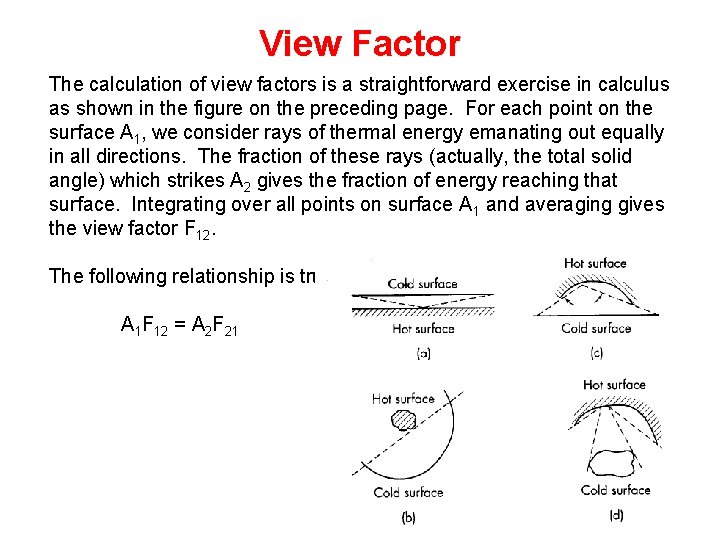
View Factor The calculation of view factors is a straightforward exercise in calculus as shown in the figure on the preceding page. For each point on the surface A 1, we consider rays of thermal energy emanating out equally in all directions. The fraction of these rays (actually, the total solid angle) which strikes A 2 gives the fraction of energy reaching that surface. Integrating over all points on surface A 1 and averaging gives the view factor F 12. The following relationship is true: A 1 F 12 = A 2 F 21

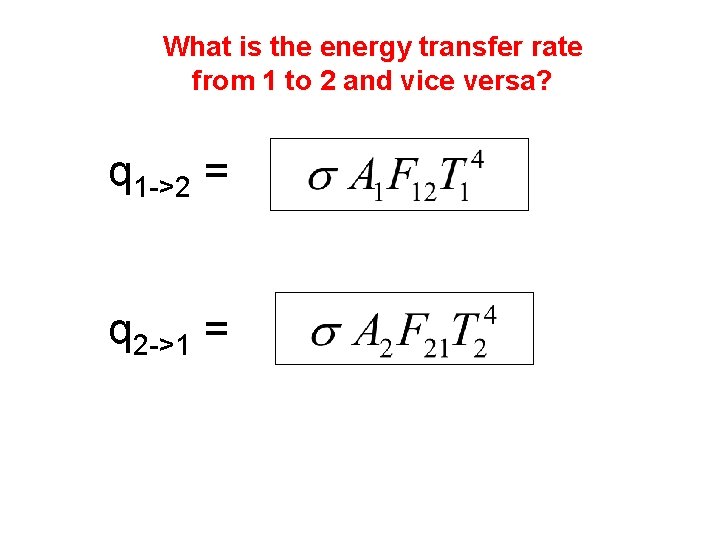
What is the energy transfer rate from 1 to 2 and vice versa? q 1 ->2 = q 2 ->1 =

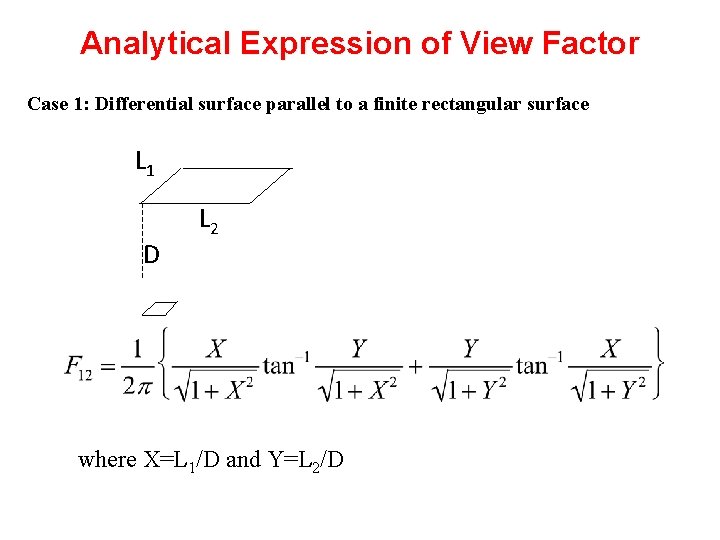
Analytical Expression of View Factor Case 1: Differential surface parallel to a finite rectangular surface L 1 D L 2 where X=L 1/D and Y=L 2/D

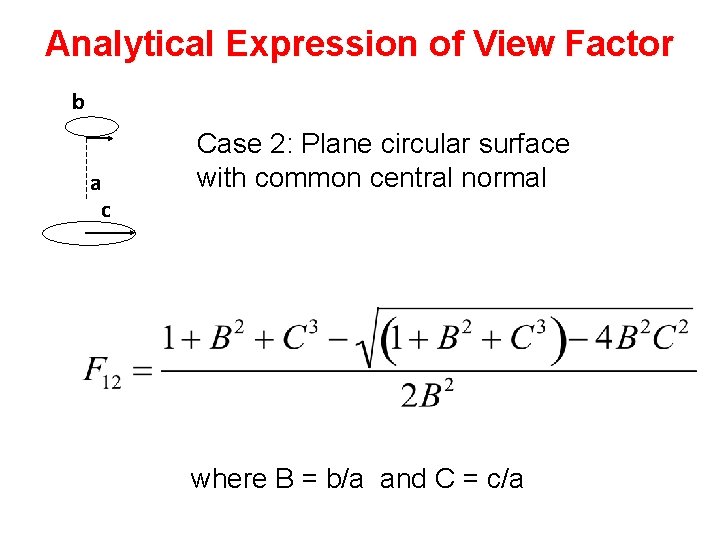
Analytical Expression of View Factor b a c Case 2: Plane circular surface with common central normal where B = b/a and C = c/a

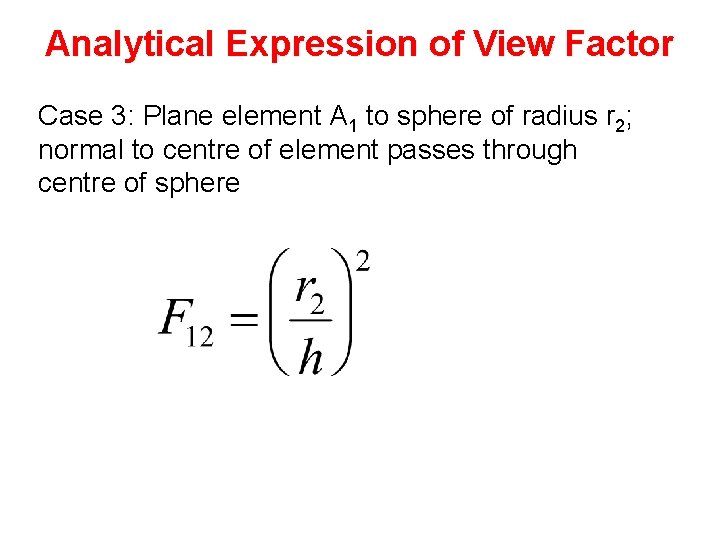
Analytical Expression of View Factor Case 3: Plane element A 1 to sphere of radius r 2; normal to centre of element passes through centre of sphere