Heat transfer in a liquid helium cooled vacuum


- Slides: 16

Heat transfer in a liquid helium cooled vacuum tube following sudden vacuum loss Ram C. Dhuley Steven W. Van Sciver National High Magnetic Field Laboratory, Tallahassee, FL 32310 Mechanical Engineering Department, FAMU-FSU College of Engineering, Tallahassee, FL 32310 June 29, 2015 | Cryogenic Engineering Conference | Tucson, AZ

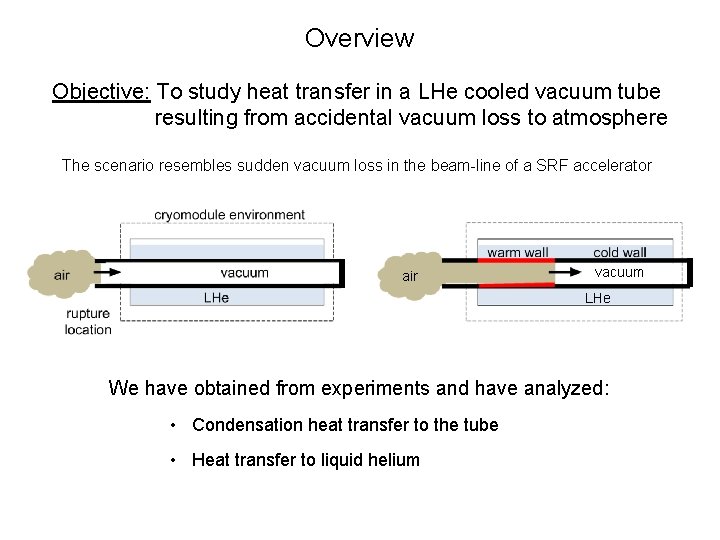
Overview Objective: To study heat transfer in a LHe cooled vacuum tube resulting from accidental vacuum loss to atmosphere The scenario resembles sudden vacuum loss in the beam-line of a SRF accelerator air vacuum LHe We have obtained from experiments and have analyzed: • Condensation heat transfer to the tube • Heat transfer to liquid helium

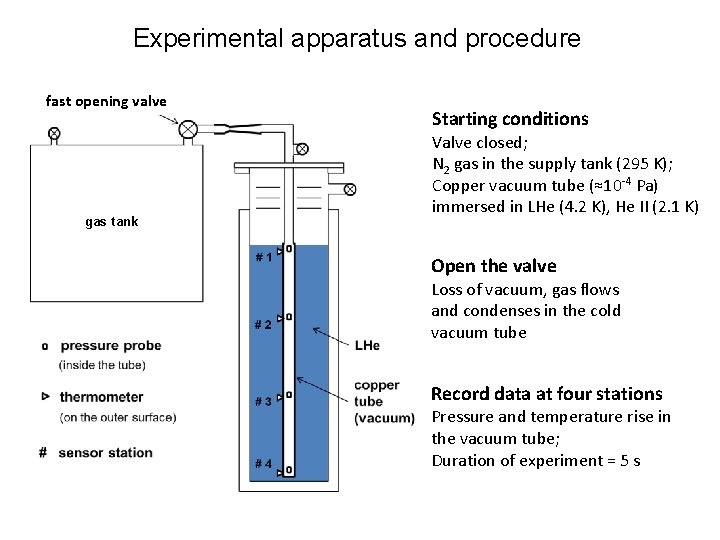
Experimental apparatus and procedure fast opening valve gas tank Starting conditions Valve closed; N 2 gas in the supply tank (295 K); Copper vacuum tube (≈10 -4 Pa) immersed in LHe (4. 2 K), He II (2. 1 K) Open the valve Loss of vacuum, gas flows and condenses in the cold vacuum tube Record data at four stations Pressure and temperature rise in the vacuum tube; Duration of experiment = 5 s

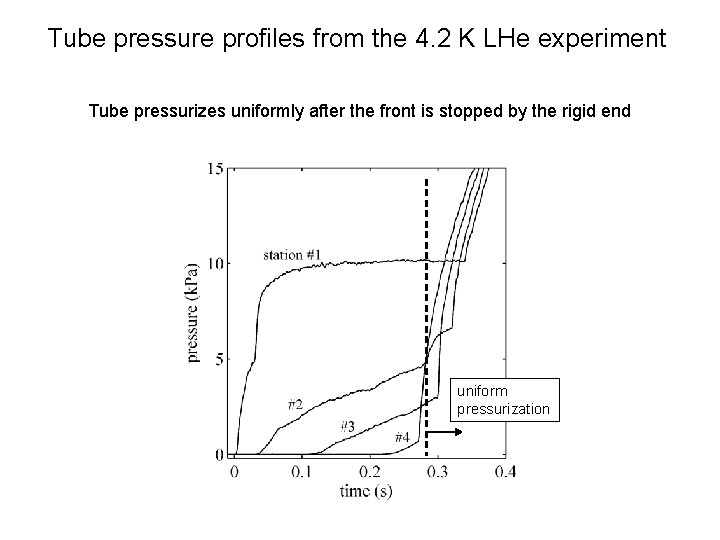
Tube pressure profiles from the 4. 2 K LHe experiment A pressure Tube pressurizes front propagates uniformly down after thethe tube front immediately is stoppedafter by the loss rigid of vacuum end uniform pressurization

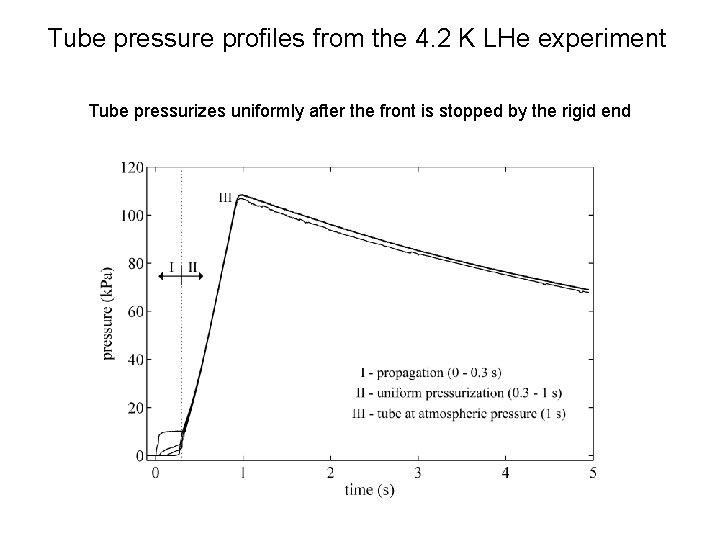
Tube pressure profiles from the 4. 2 K LHe experiment A pressure Tube As pressurizes front more propagates gasuniformly flows down in, the after the tube pressurizes front immediately is stopped to atmosphere after by the loss rigid of vacuum end

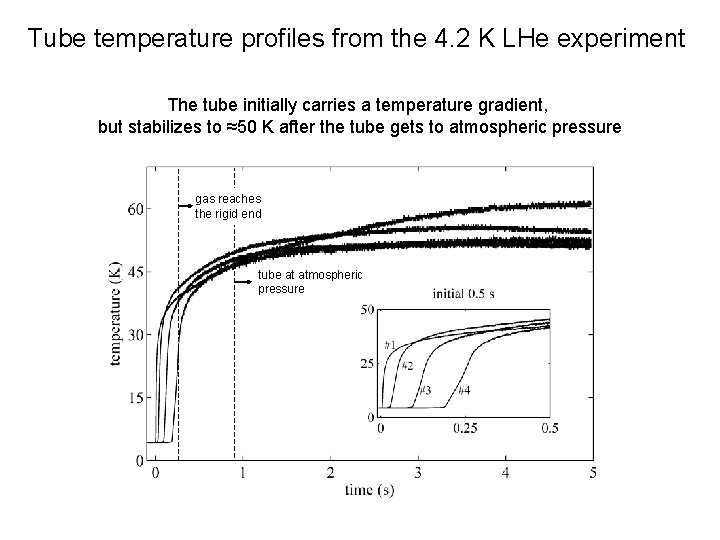
Tube temperature profiles from the 4. 2 K LHe experiment The tube initially carries a temperature gradient, but stabilizes to ≈50 K after the tube gets to atmospheric pressure gas reaches the rigid end tube at atmospheric pressure

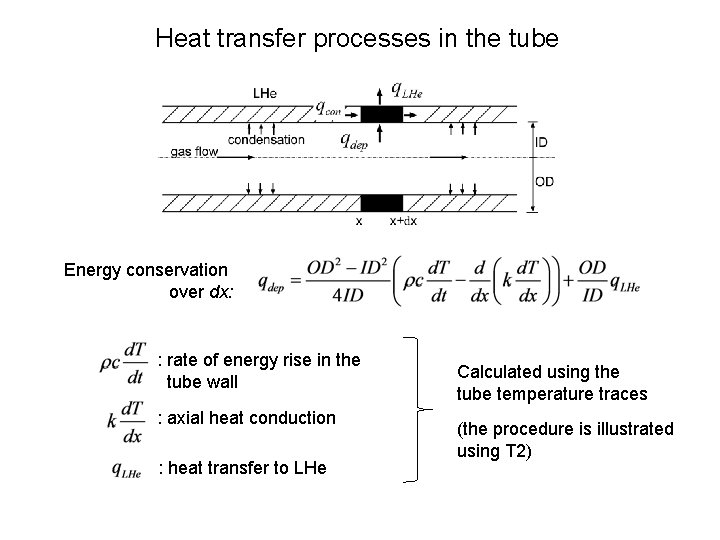
Heat transfer processes in the tube Energy conservation over dx: : rate of energy rise in the tube wall : axial heat conduction : heat transfer to LHe Calculated using the tube temperature traces (the procedure is illustrated using T 2)

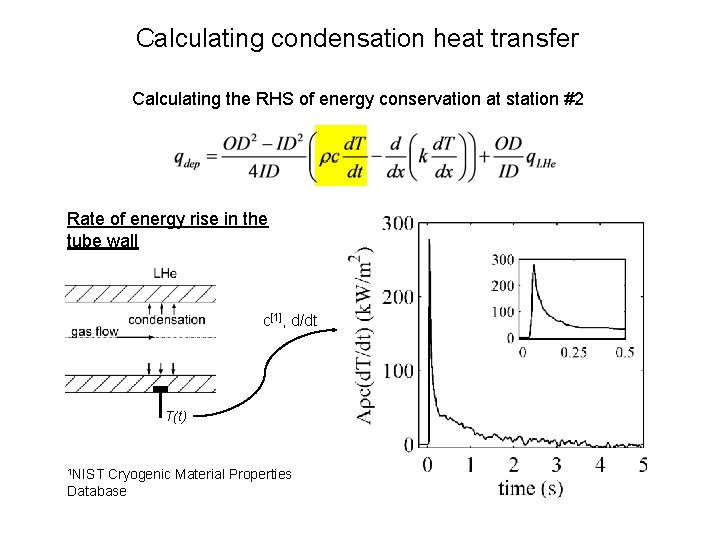
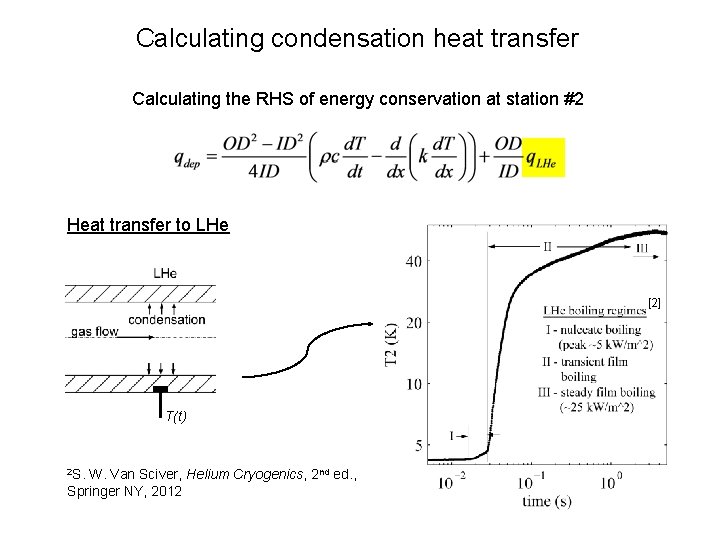
Calculating condensation heat transfer Calculating the RHS of energy conservation at station #2 Rate of energy rise in the tube wall c[1], d/dt T(t) 1 NIST Cryogenic Material Properties Database

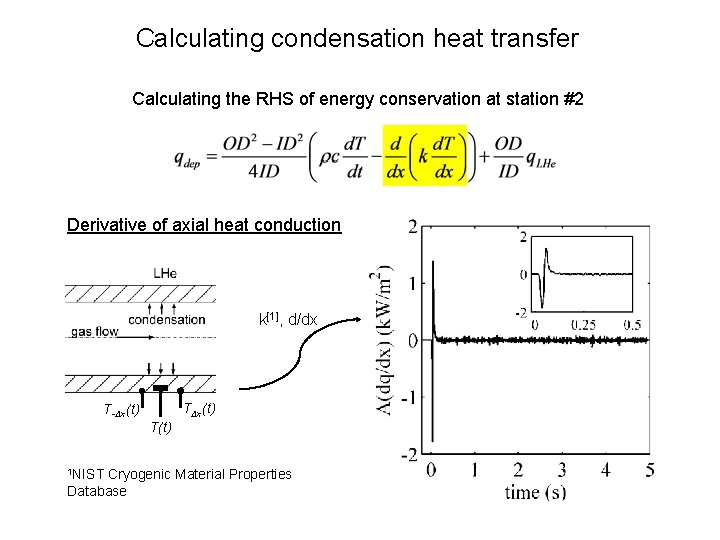
Calculating condensation heat transfer Calculating the RHS of energy conservation at station #2 Derivative of axial heat conduction k[1], d/dx TΔx(t) T-Δx(t) T(t) 1 NIST Cryogenic Material Properties Database

Calculating condensation heat transfer Calculating the RHS of energy conservation at station #2 Heat transfer to LHe [2] T(t) 2 S. W. Van Sciver, Helium Cryogenics, 2 nd ed. , Springer NY, 2012

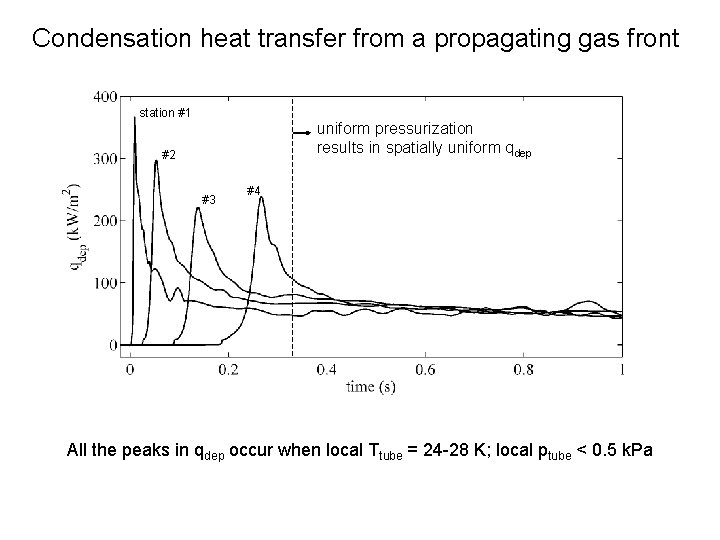
Condensation heat transfer from a propagating gas front station #1 Two competing processes uniform pressurization results in- spatially uniform qdep I) Rising pressure > faster condensation #2 II) Rising tube temperature - > slower condensation #3 #4 I << II I >> II All the peaks in qdep occur when local Ttube = 24 -28 K; local ptube < 0. 5 k. Pa

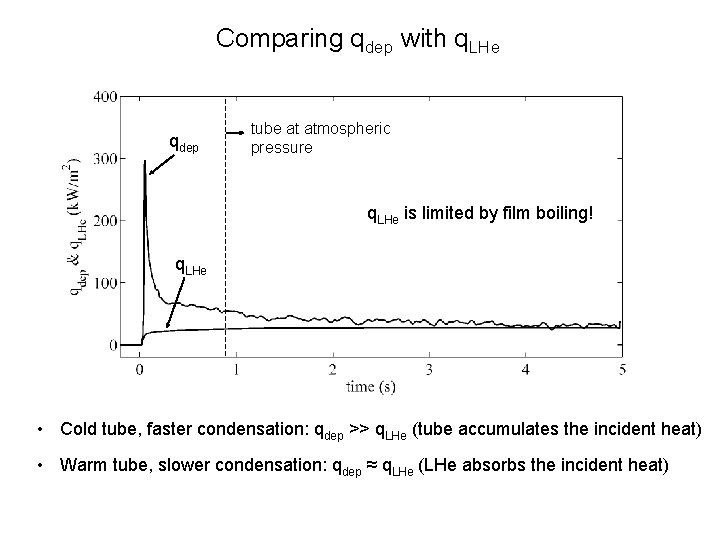
Comparing qdep with q. LHe qdep tube at atmospheric pressure q. LHe is limited by film boiling! q. LHe ≈ 25 k. W/m 2 • Cold tube, faster condensation: qdep >> q. LHe (tube accumulates the incident heat) • Warm tube, slower condensation: qdep ≈ q. LHe (LHe absorbs the incident heat)

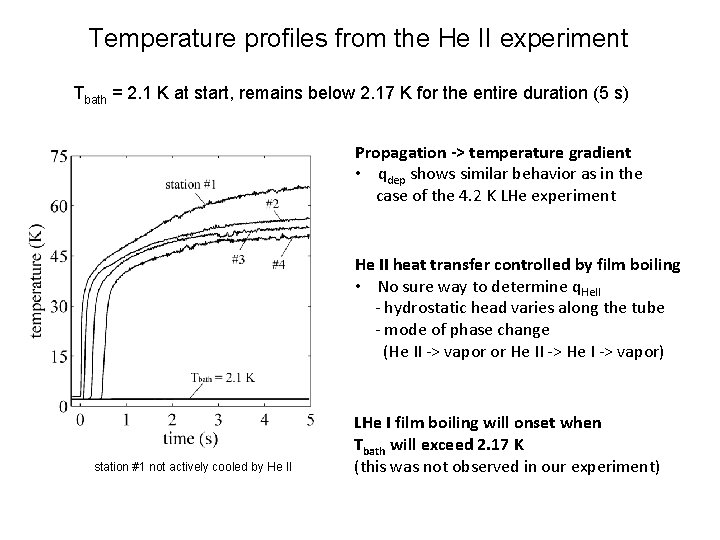
Temperature profiles from the He II experiment Tbath = 2. 1 K at start, remains below 2. 17 K for the entire duration (5 s) * Propagation -> temperature gradient • qdep shows similar behavior as in the case of the 4. 2 K LHe experiment He II heat transfer controlled by film boiling • No sure way to determine q. He. II - hydrostatic head varies along the tube - mode of phase change (He II -> vapor or He II -> He I -> vapor) station #1 not actively cooled by He II LHe I film boiling will onset when Tbath will exceed 2. 17 K (this was not observed in our experiment)

Conclusions • A gas pressure front propagates in the tube following sudden vacuum loss • Condensation heat transfer to the tube is largely controlled by the tube temperature - highest when the tube temperature is in the 24 -28 K range - rapidly drops as the tube warms above this temperature • High instantaneous heat fluxes (>200 k. W/m 2) are deposited on to the tube by the propagating pressure front • Heat transfer to LHe is limited by film boiling

Acknowledgement • Department of Energy Grant DE-FG 02 -96 ER 40952 • Dr. Wei Guo and Dr. Ernesto Bosque of NHMFL-FSU • Colleagues at NHMFL Cryogenics lab - Dr. Mark Vanderlaan, Jian Gao, Brian Mastracci, and Andrew Wray • NHMFL is supported by the US National Science Foundation and the State of Florida.

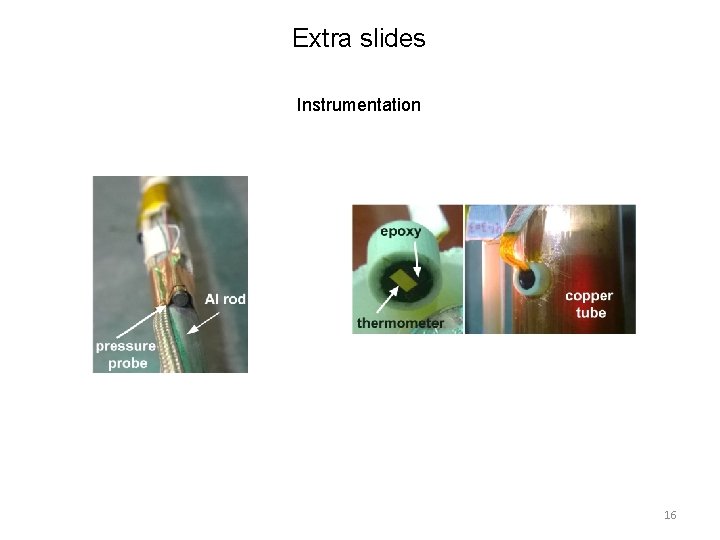
Extra slides Instrumentation 16