Heat Transfer First Law of Thermodynamics PHYS116 A03




- Slides: 20

Heat Transfer, First Law of Thermodynamics PHYS-116 A-03 11/30/2012 Lecture 30 Momchil Velkovsky

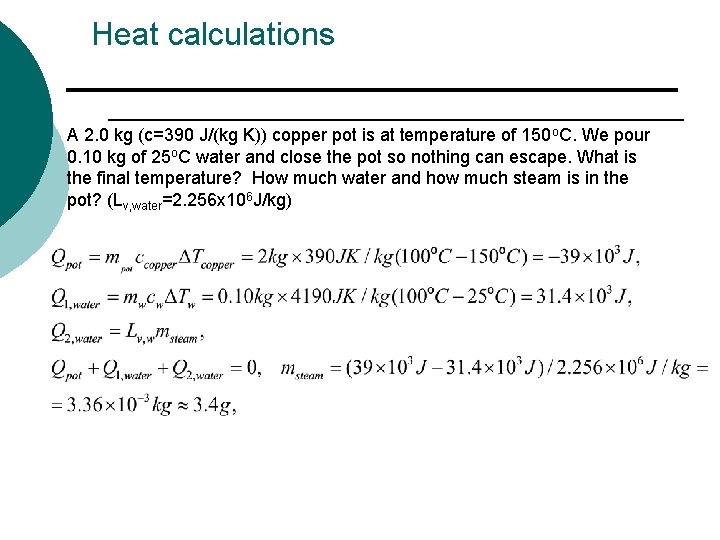
Heat calculations A 2. 0 kg (c=390 J/(kg K)) copper pot is at temperature of 150 o. C. We pour 0. 10 kg of 25 o. C water and close the pot so nothing can escape. What is the final temperature? How much water and how much steam is in the pot? (Lv, water=2. 256 x 106 J/kg)

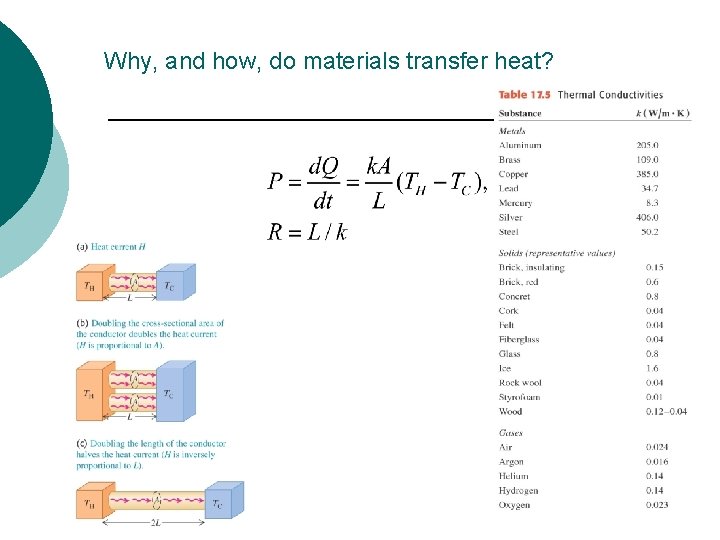
Why, and how, do materials transfer heat?

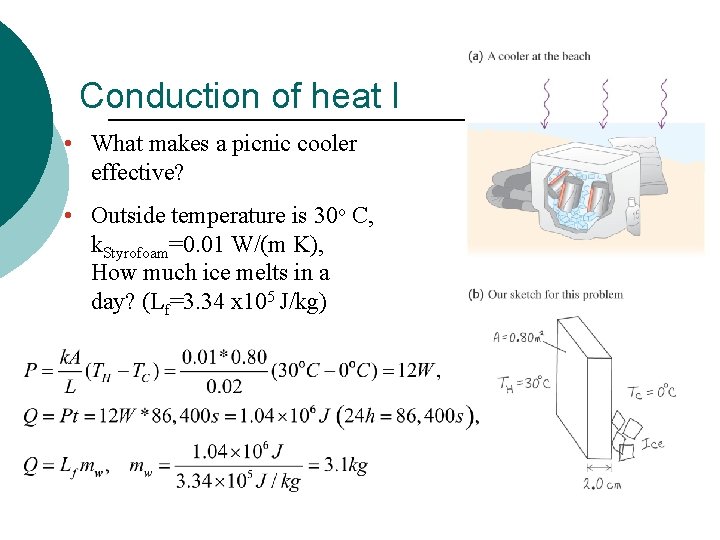
Conduction of heat I • What makes a picnic cooler effective? • Outside temperature is 30 o C, k. Styrofoam=0. 01 W/(m K), How much ice melts in a day? (Lf=3. 34 x 105 J/kg)

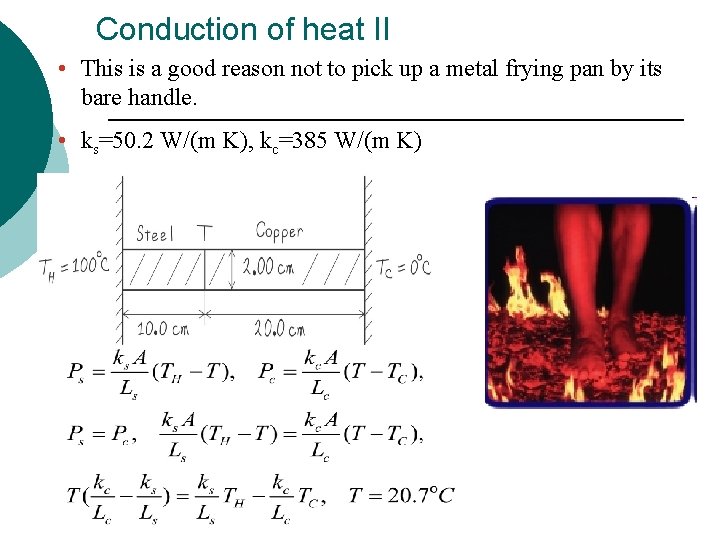
Conduction of heat II • This is a good reason not to pick up a metal frying pan by its bare handle. • ks=50. 2 W/(m K), kc=385 W/(m K)

A chair has a wooden seat but metal legs. The chair legs feel colder to the touch than does the seat. Why is this? ¡ ¡ ¡ A. The metal is at a lower temperature than the wood. B. The metal has a higher specific heat than the wood. C. The metal has a lower specific heat than the wood. D. The metal has a higher thermal conductivity than the wood. E. The metal has a lower thermal conductivity than the wood.

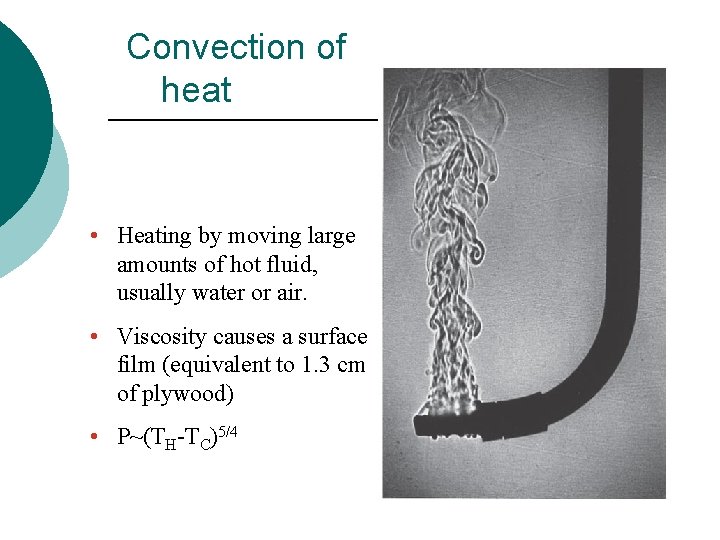
Convection of heat • Heating by moving large amounts of hot fluid, usually water or air. • Viscosity causes a surface film (equivalent to 1. 3 cm of plywood) • P~(TH-TC)5/4

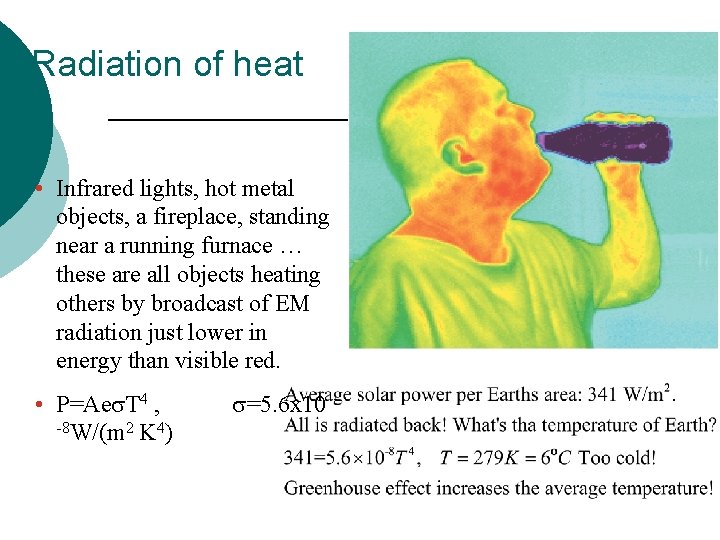
Radiation of heat • Infrared lights, hot metal objects, a fireplace, standing near a running furnace … these are all objects heating others by broadcast of EM radiation just lower in energy than visible red. • P=Aes. T 4 , -8 W/(m 2 K 4) s=5. 6 x 10

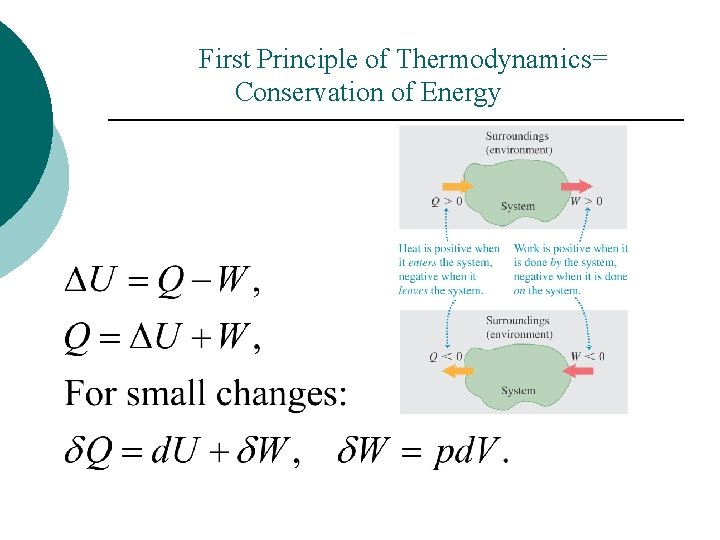
First Principle of Thermodynamics= Conservation of Energy

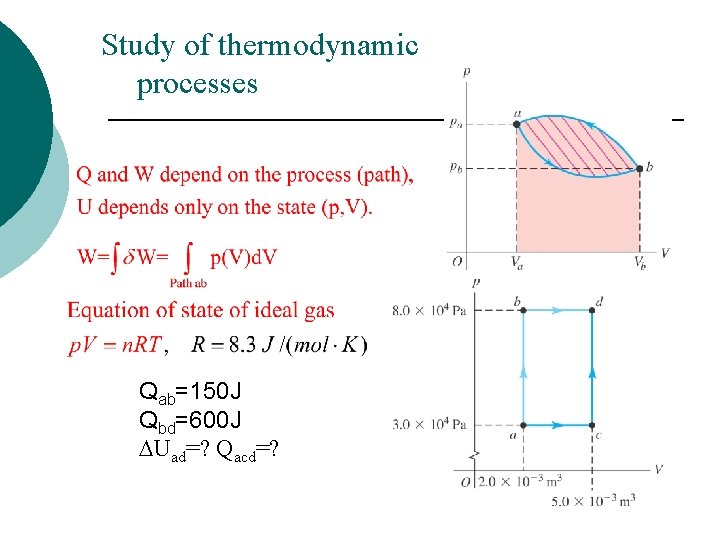
Study of thermodynamic processes Qab=150 J Qbd=600 J DUad=? Qacd=?

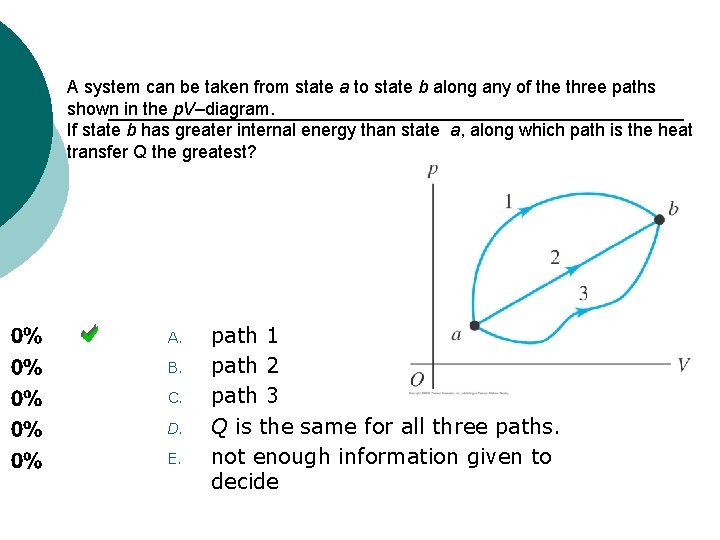
A system can be taken from state a to state b along any of the three paths shown in the p. V–diagram. If state b has greater internal energy than state a, along which path is the heat transfer Q the greatest? A. B. C. D. E. path 1 path 2 path 3 Q is the same for all three paths. not enough information given to decide

A gas is enclosed in a cylinder by a piston. The volume of the gas is then reduced to one half its original value by applying a force to the piston. Which one of the following statements is true? ¡ ¡ ¡ a) The internal energy of the gas will decrease. b) The internal energy of the gas will increase. c) The internal energy of the gas will neither increase nor decrease. d) The internal energy of the gas will equal the work done in moving the piston. e) The internal energy of the gas may increase, decrease, or remain the same

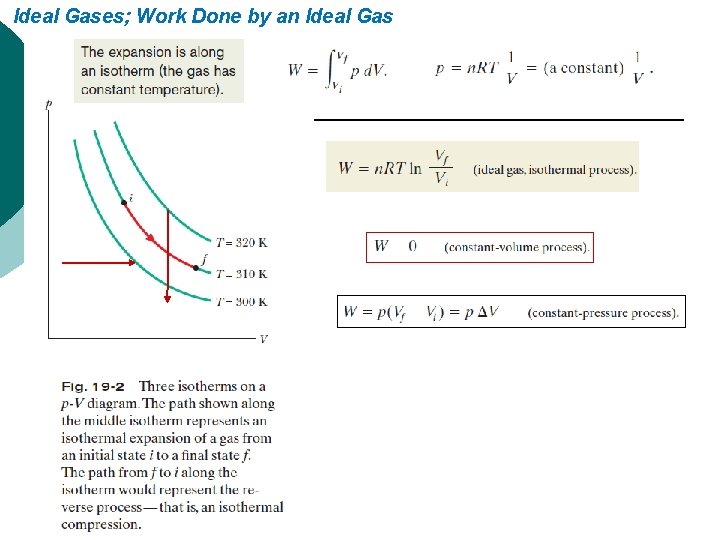
Ideal Gases; Work Done by an Ideal Gas

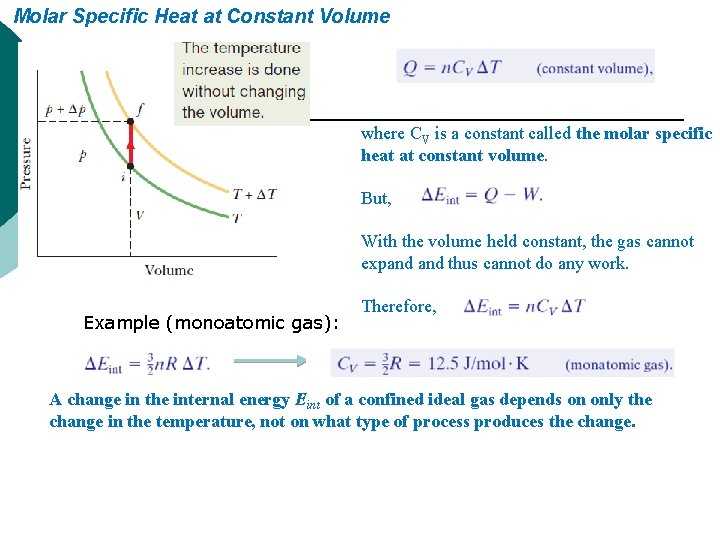
Molar Specific Heat at Constant Volume where CV is a constant called the molar specific heat at constant volume. But, With the volume held constant, the gas cannot expand thus cannot do any work. Example (monoatomic gas): Therefore, A change in the internal energy Eint of a confined ideal gas depends on only the change in the temperature, not on what type of process produces the change.

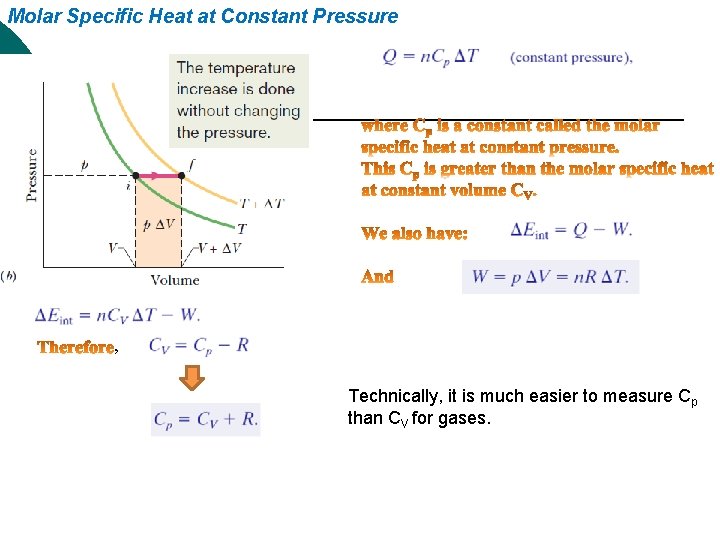
Molar Specific Heat at Constant Pressure , Technically, it is much easier to measure Cp than CV for gases.

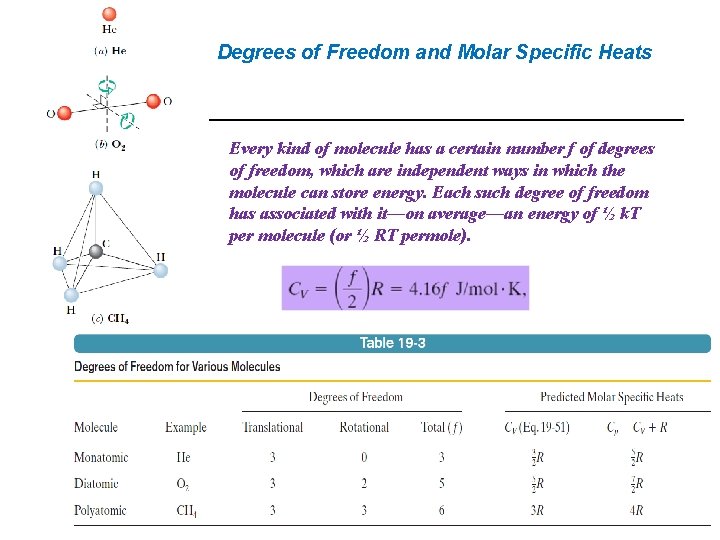
Degrees of Freedom and Molar Specific Heats Every kind of molecule has a certain number f of degrees of freedom, which are independent ways in which the molecule can store energy. Each such degree of freedom has associated with it—on average—an energy of ½ k. T per molecule (or ½ RT permole).

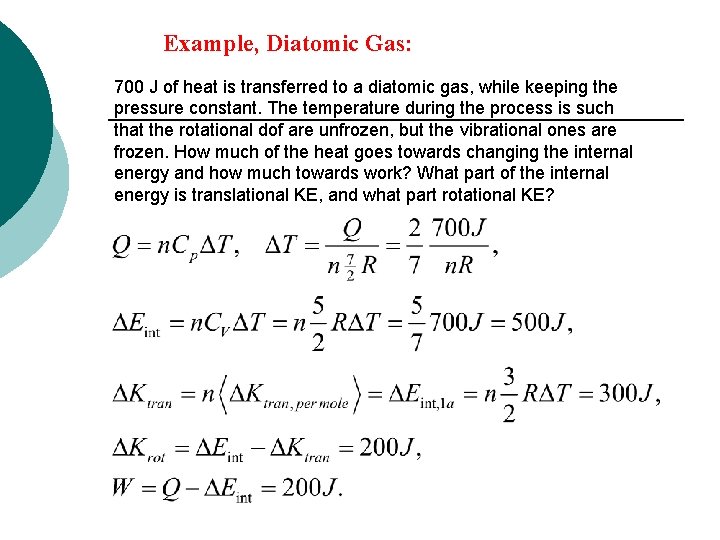
Example, Diatomic Gas: 700 J of heat is transferred to a diatomic gas, while keeping the pressure constant. The temperature during the process is such that the rotational dof are unfrozen, but the vibrational ones are frozen. How much of the heat goes towards changing the internal energy and how much towards work? What part of the internal energy is translational KE, and what part rotational KE?

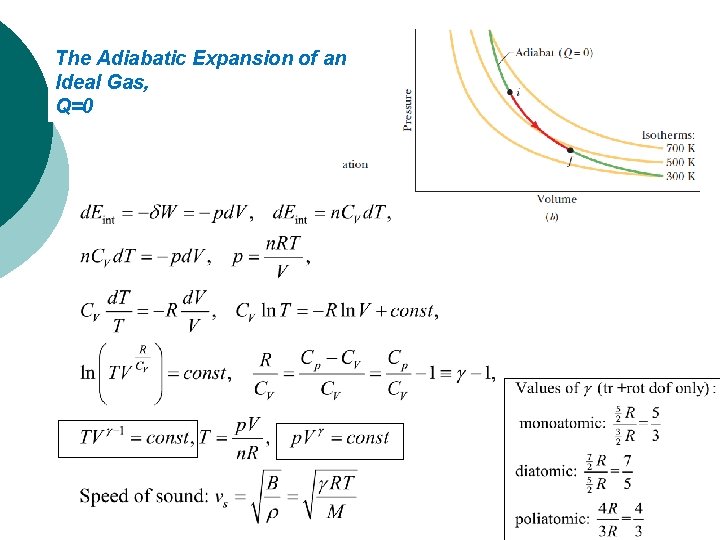
The Adiabatic Expansion of an Ideal Gas, Q=0

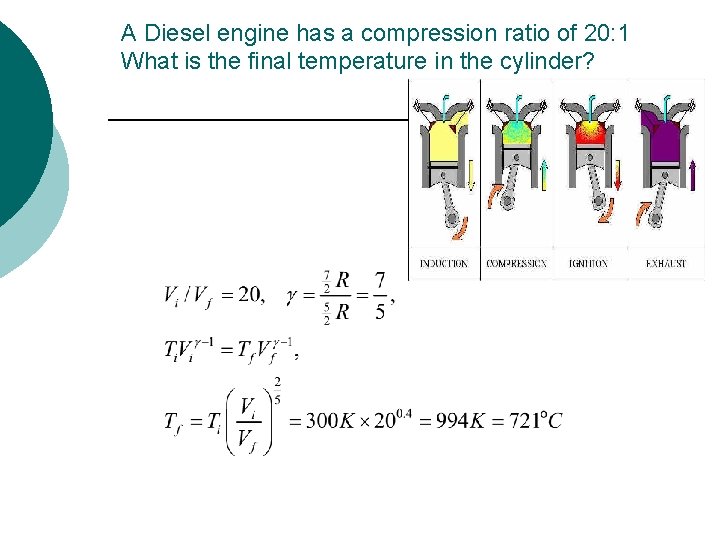
A Diesel engine has a compression ratio of 20: 1 What is the final temperature in the cylinder?

Next time ¡ Read 18 -9 18 -12