Heat Transfer EQ What are three ways heat








- Slides: 36

Heat Transfer EQ: What are three ways heat is transferred?

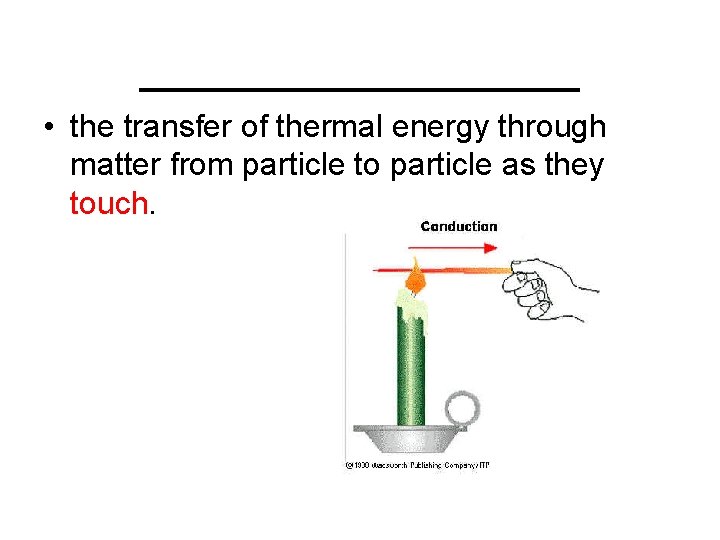
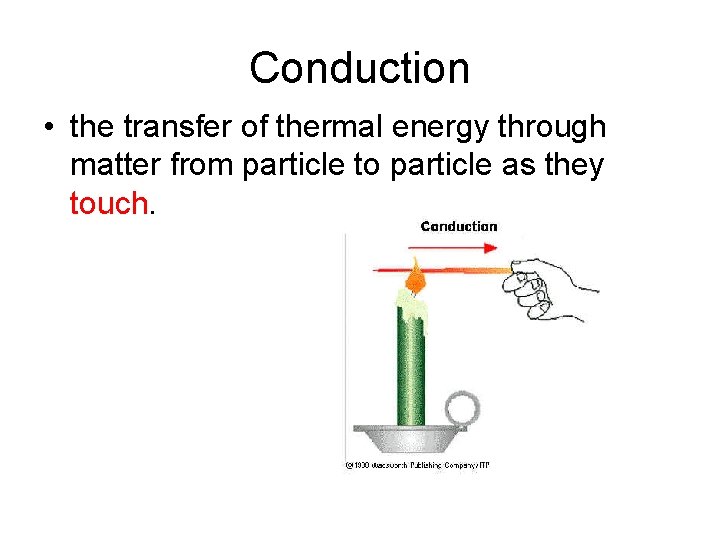
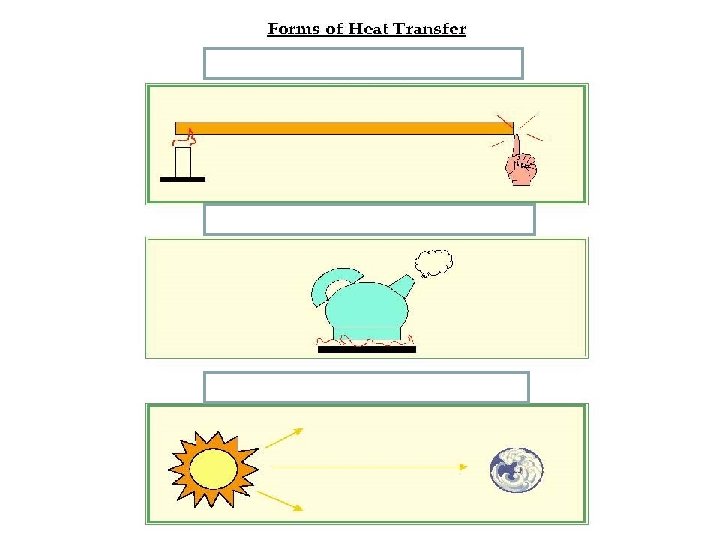
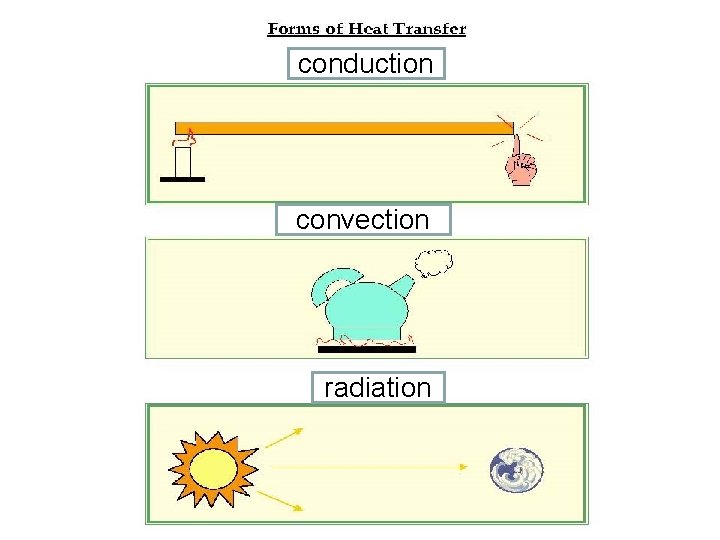
_________ • the transfer of thermal energy through matter from particle to particle as they touch.

Conduction • the transfer of thermal energy through matter from particle to particle as they touch.

A spoon in a cup of hot soup becomes warmer because the heat from the soup is conducted along the spoon. Conduction is most effective in solids-but it can happen in fluids (liquids and gases).

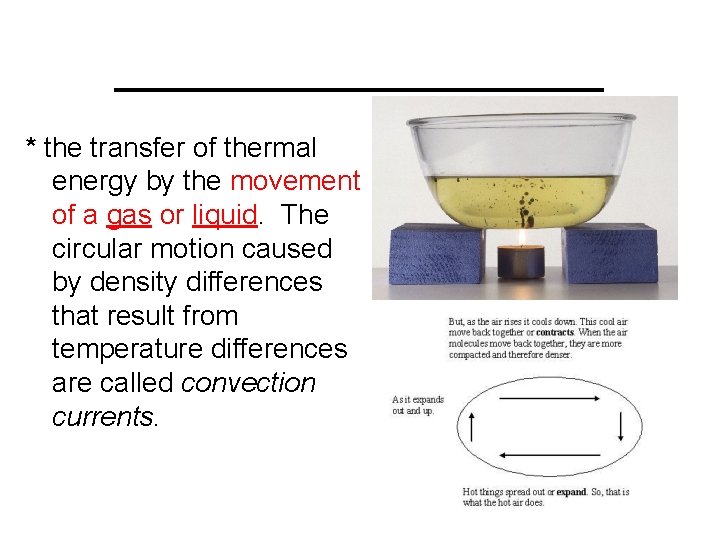
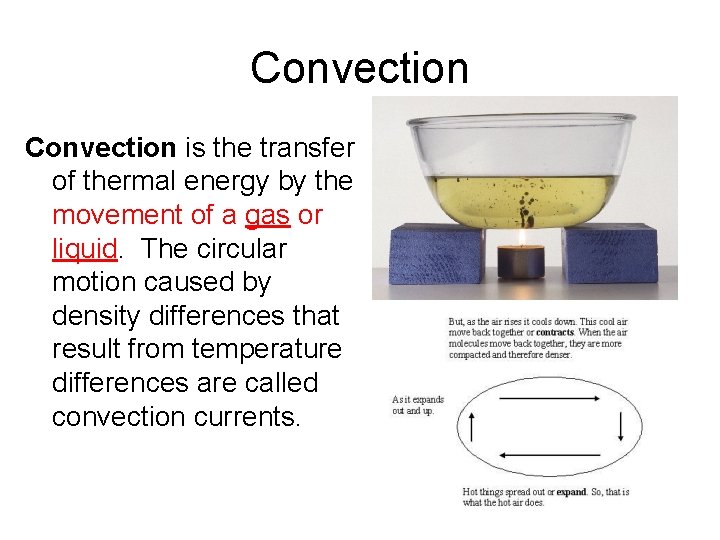
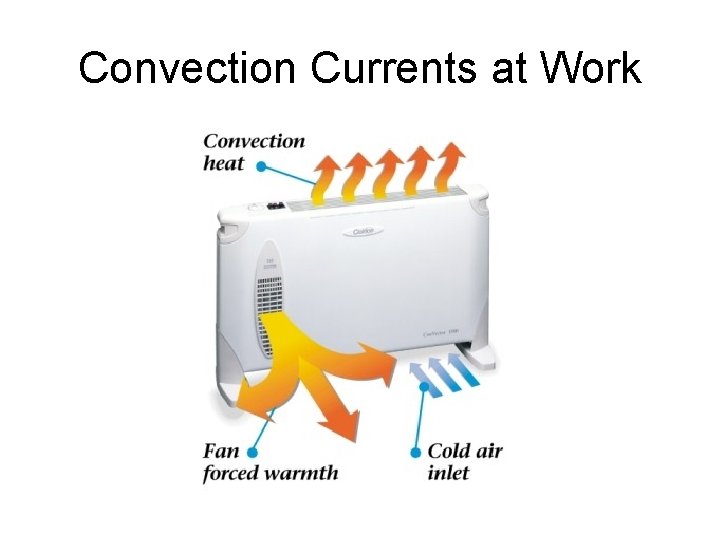
__________ * the transfer of thermal energy by the movement of a gas or liquid. The circular motion caused by density differences that result from temperature differences are called convection currents.

Convection is the transfer of thermal energy by the movement of a gas or liquid. The circular motion caused by density differences that result from temperature differences are called convection currents.

Convection Currents at Work

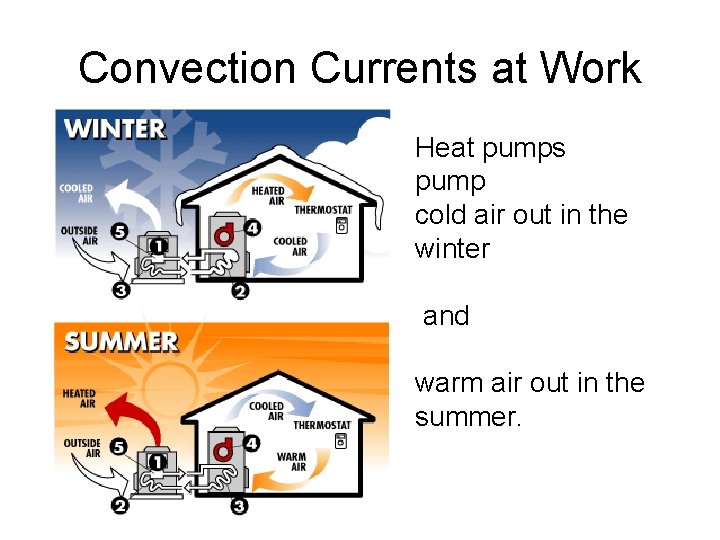
Convection Currents at Work Heat pumps pump cold air out in the winter and warm air out in the summer.

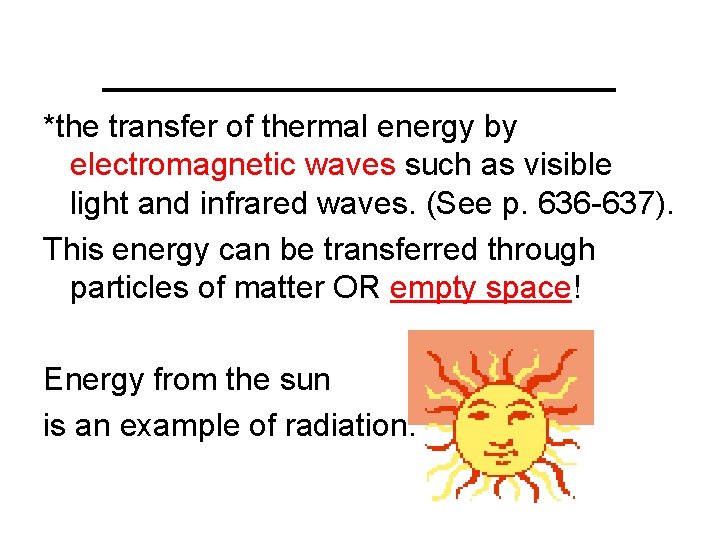
___________ *the transfer of thermal energy by electromagnetic waves such as visible light and infrared waves. (See p. 636 -637). This energy can be transferred through particles of matter OR empty space! Energy from the sun is an example of radiation.

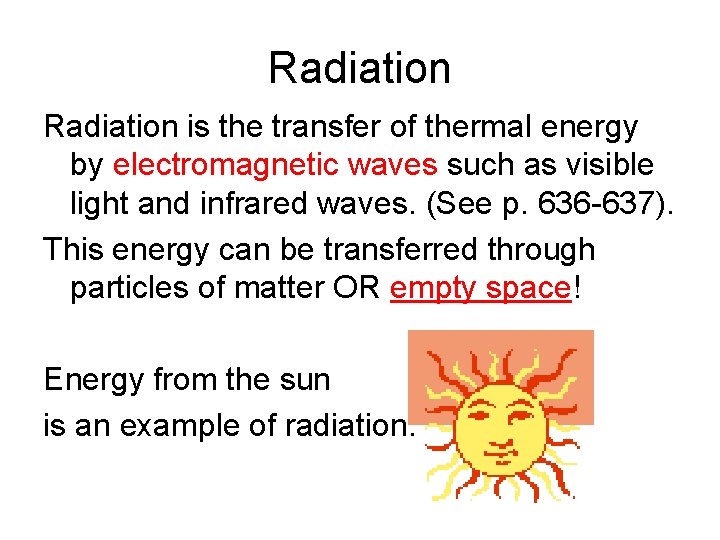
Radiation is the transfer of thermal energy by electromagnetic waves such as visible light and infrared waves. (See p. 636 -637). This energy can be transferred through particles of matter OR empty space! Energy from the sun is an example of radiation.

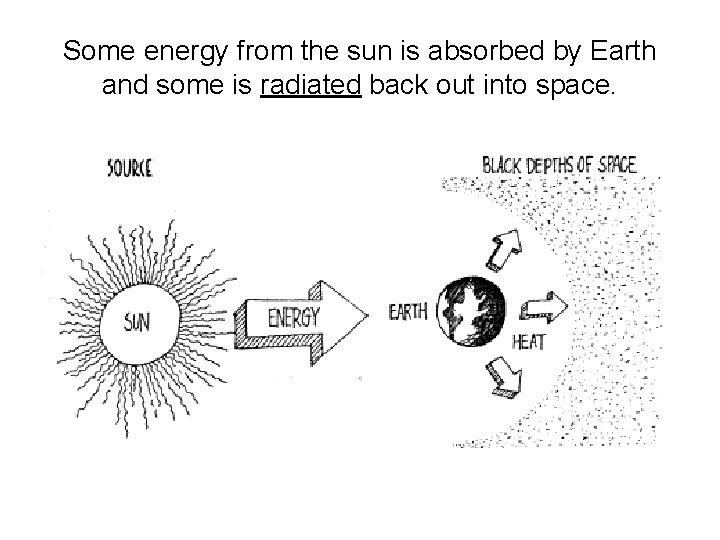
Some energy from the sun is absorbed by Earth and some is radiated back out into space.

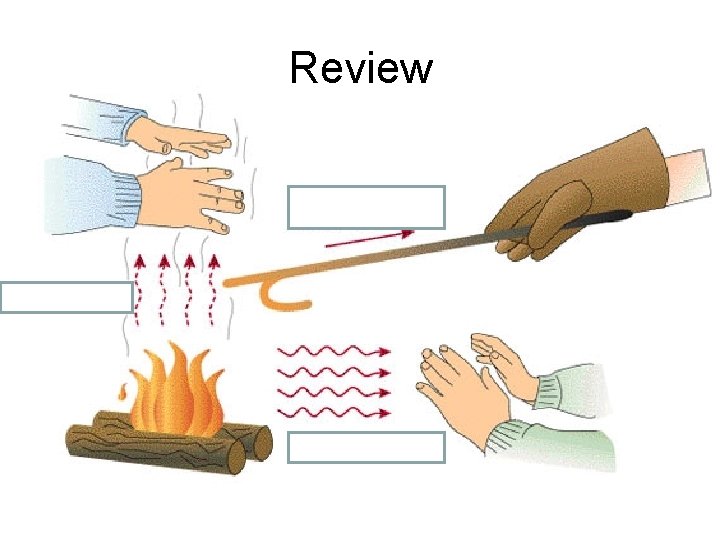
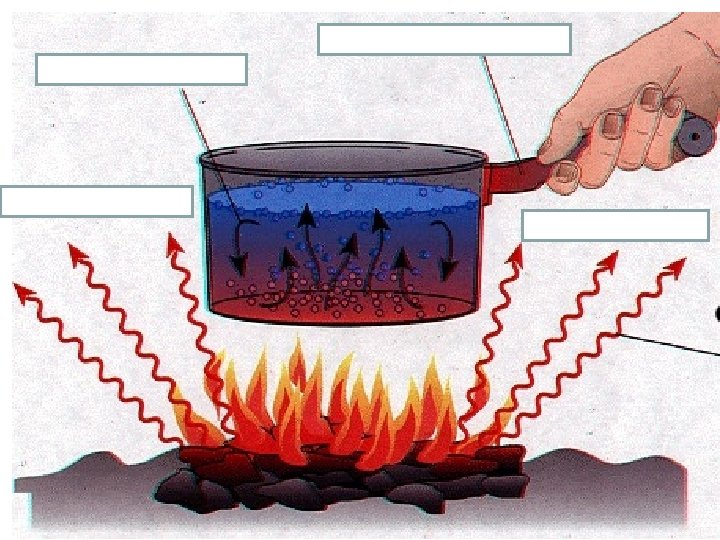
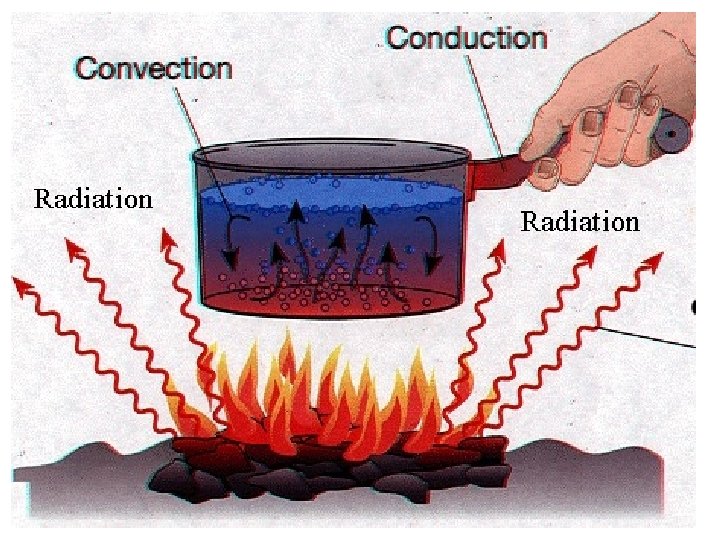
Review

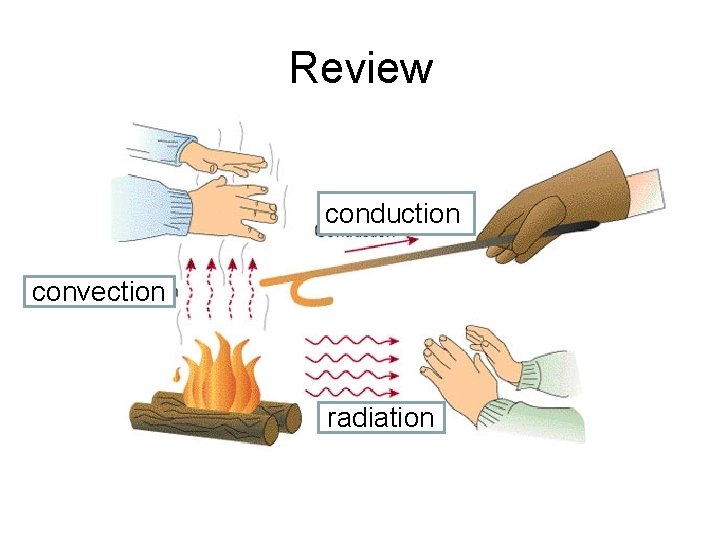
Review conduction convection radiation

Review

Review conduction convection radiation



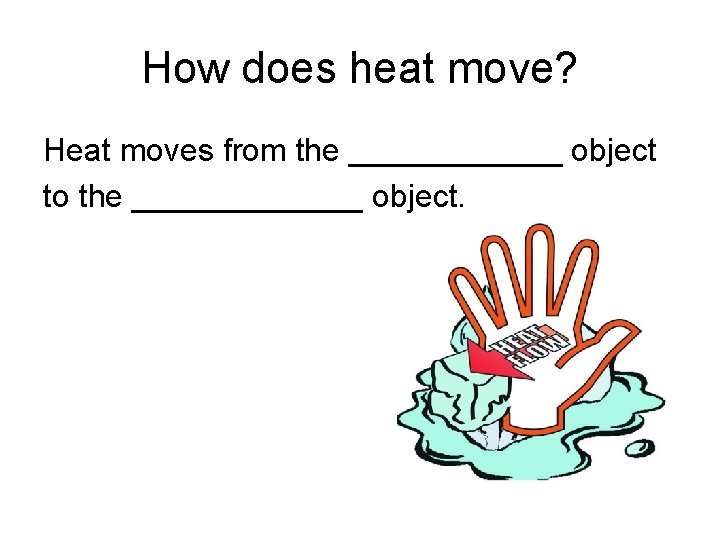
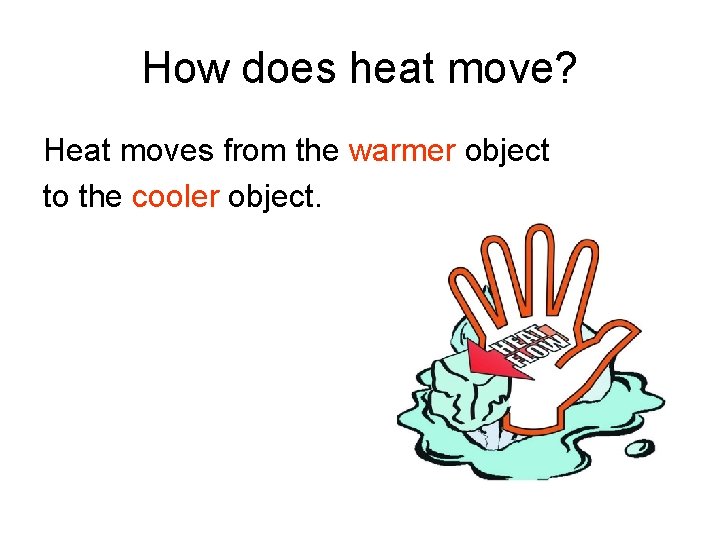
How does heat move? Heat moves from the ______ object to the _______ object.

How does heat move? Heat moves from the warmer object to the cooler object.

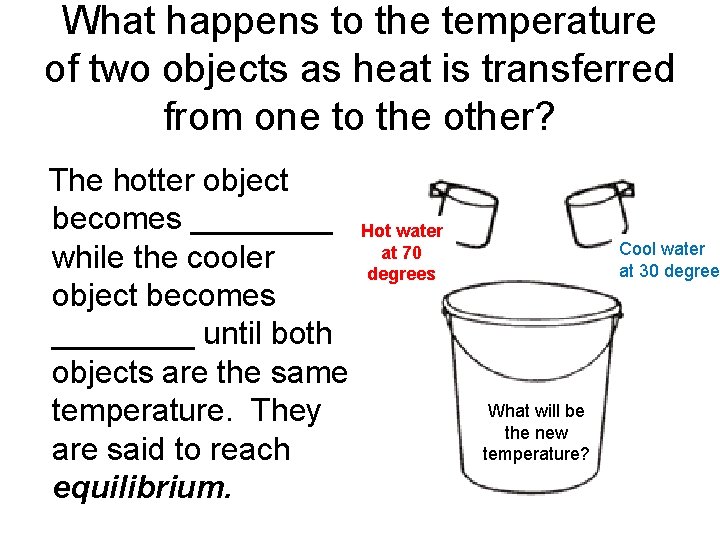
What happens to the temperature of two objects as heat is transferred from one to the other? The hotter object becomes ____ while the cooler object becomes ____ until both objects are the same temperature. They are said to reach equilibrium. Hot water at 70 degrees Cool water at 30 degree What will be the new temperature?

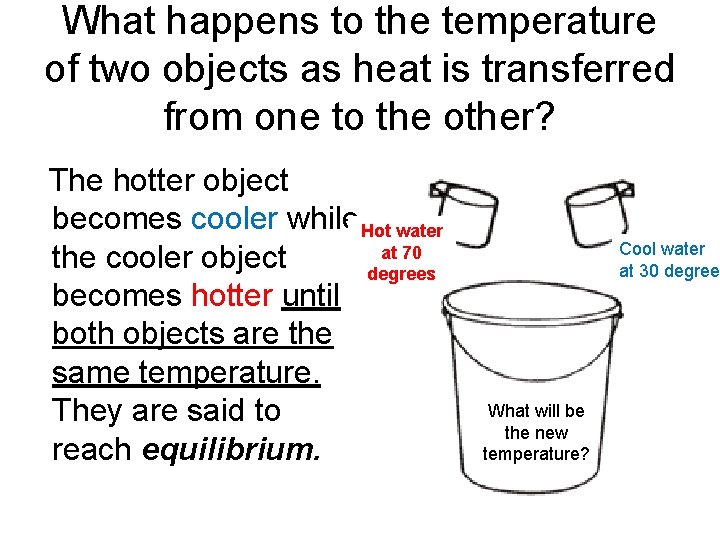
What happens to the temperature of two objects as heat is transferred from one to the other? The hotter object becomes cooler while Hot water at 70 the cooler object degrees becomes hotter until both objects are the same temperature. They are said to reach equilibrium. Cool water at 30 degree What will be the new temperature?

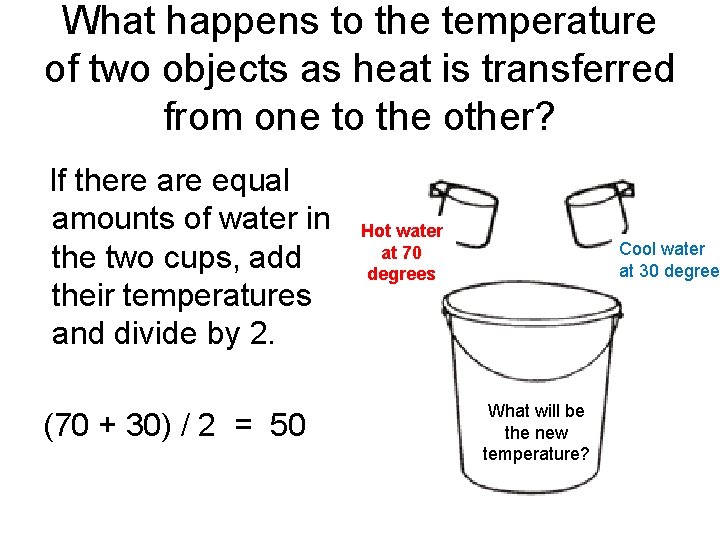
What happens to the temperature of two objects as heat is transferred from one to the other? If there are equal amounts of water in the two cups, add their temperatures and divide by 2. (70 + 30) / 2 = 50 Hot water at 70 degrees Cool water at 30 degree What will be the new temperature?

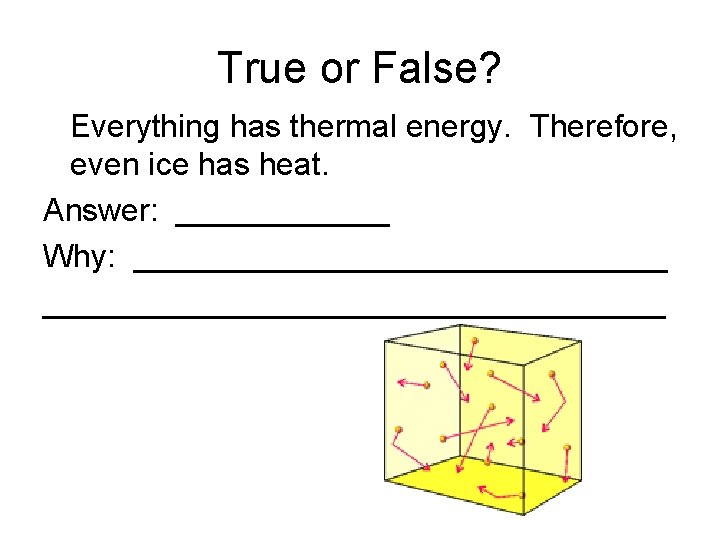
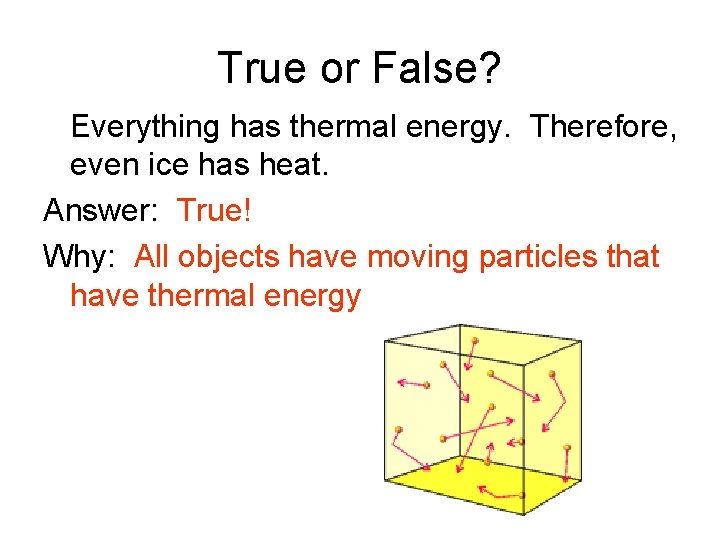
True or False? Everything has thermal energy. Therefore, even ice has heat. Answer: ______ Why: ___________________________________

True or False? Everything has thermal energy. Therefore, even ice has heat. Answer: True! Why: All objects have moving particles that have thermal energy

True or False? A swimming pool has more thermal energy than a pot of hot coffee. Answer: __________ Why: __________________________________

True or False? A swimming pool has more thermal energy than a pot of hot coffee. Answer: True Why: A swimming pool has many more particles than a pot of coffee—each with energy (Thermal energy = TOTAL energy)

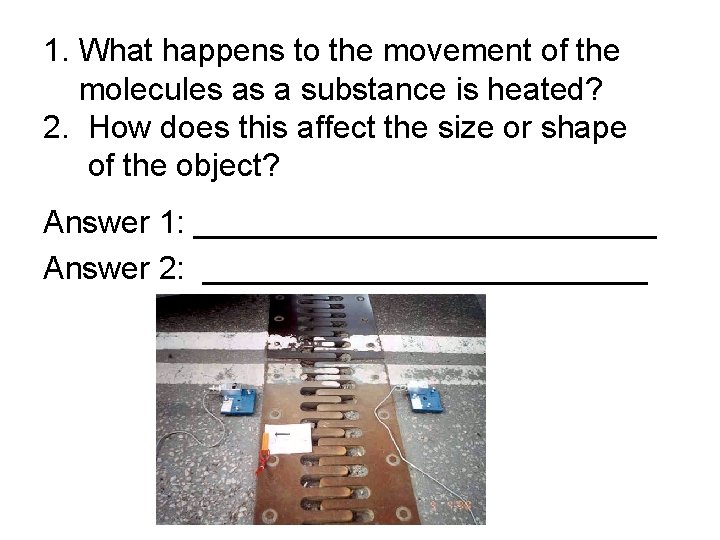
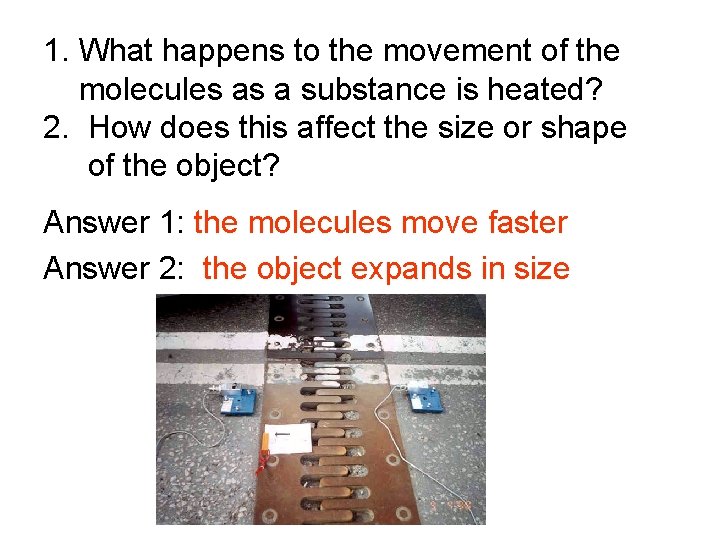
1. What happens to the movement of the molecules as a substance is heated? 2. How does this affect the size or shape of the object? Answer 1: _____________ Answer 2: _____________

1. What happens to the movement of the molecules as a substance is heated? 2. How does this affect the size or shape of the object? Answer 1: the molecules move faster Answer 2: the object expands in size

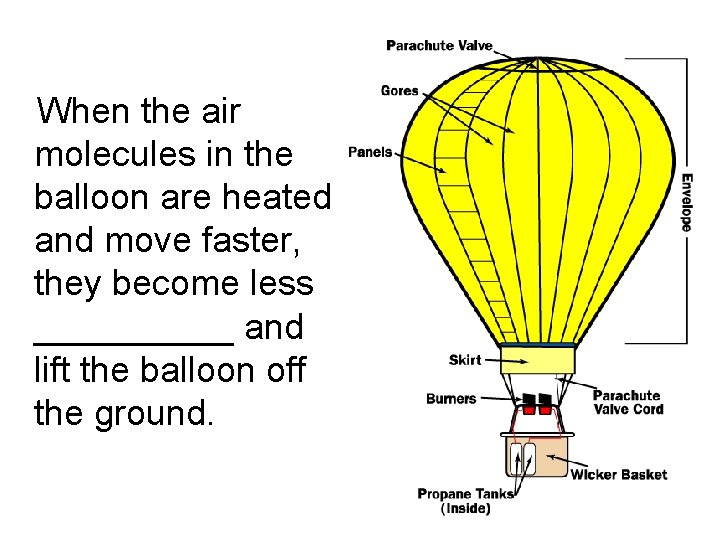
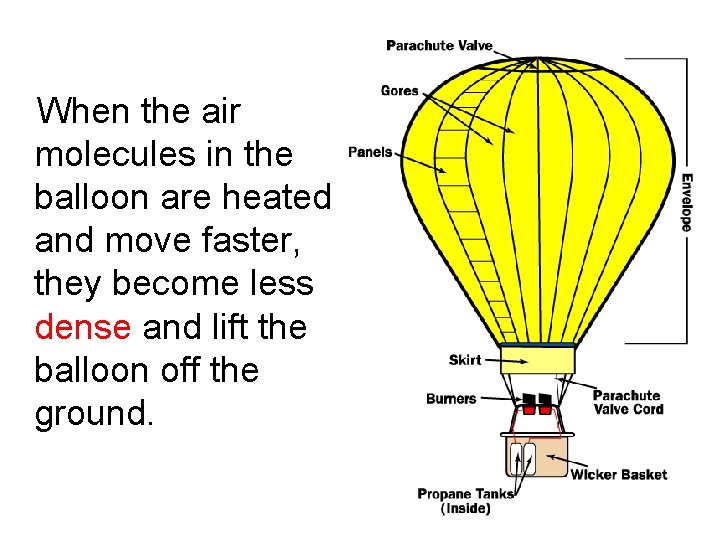
When the air molecules in the balloon are heated and move faster, they become less _____ and lift the balloon off the ground.

When the air molecules in the balloon are heated and move faster, they become less dense and lift the balloon off the ground.

Why do some objects feel cold to the touch while others feel warm? Answer: ___________________________________

Why do some objects feel cold to the touch while others feel warm? Answer: Objects may FEEL cold because heat is conducted quickly away from the body (conductors) or they may FEEL warm because heat is conducted slowly away from the body (insulators).

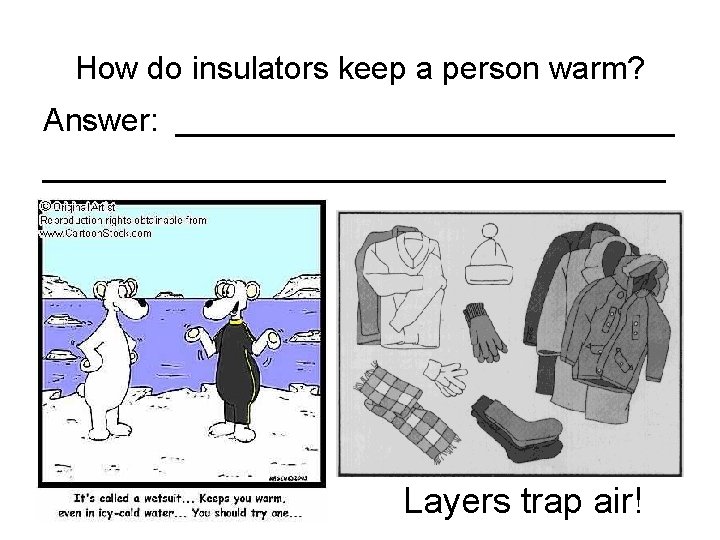
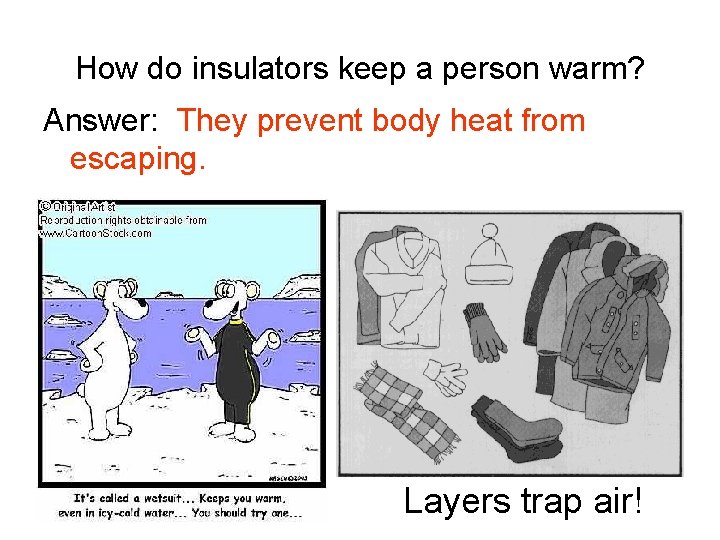
How do insulators keep a person warm? Answer: ___________________________________ Layers trap air!

How do insulators keep a person warm? Answer: They prevent body heat from escaping. Layers trap air!

Air is a poor conductor. Therefore, it is a good insulator.

Review Game Click here to review what you have learned. Number your paper 1 -20 and see how you do. Good luck!