Heat Temperature Specific Heat Thermal Energy Total energy










- Slides: 27

Heat, Temperature & Specific Heat

Thermal Energy �Total energy in molecules of a substance including �a) kinetic E of moving molecules �b) potential E stored in chemical bonds

Temperature = �Average kinetic energy per molecule �Average speed of molecule �KE = ½ m V 2 �Velocity � 0 m = mass V = velocity or speed is most important velocity = absolute 0…. no motion of particles

Heat = �Flow of thermal Energy from hot to cold �Transfer of kinetic energy from one molecule to another � http: //www. gcse. com/energy/images/conduction. gif

Laws of Thermodynamics � 1) Energy can not be created or destroyed �only converted from one form to another �or transferred from one place to another � 2) Heat energy flows from high temperature areas to low temperature areas only

Thermal equilibrium �No net flow of heat energy because all areas are the same temperature �Heat flows from hot to cold until…. �Thermal equilibrium is reached…. �Then it just passes back and forth �Still moving so still heat!

Thermometers… �Measure their own temperature �Put thermometer in water. Heat flows �Molecules of alcohol speed up and expand or �slow down and contract �Thermal equilibrium between thermometer and water causes red line to stop moving. � http: //www. middleschoolchemistry. com/multimedia/chapter 1/lesson 3#heating_and_cooling_a_thermometer

Measurments & Units �Temperature is measured in… o � �Heat is measures in…. calories � � 1 � � � Celsius calorie = the amount of heat needed to raise the temp of 1 ml H 2 O by 1 o C

�The Calories in your food are Calories, �They � are actually Kilocalories = the amount of heat needed to warm up 1, 000 ml of H 2 O by 1 o. C

Specific Heat �It takes more heat energy to increase the temperature of some substances. �We say those substances have a high specific heat. �Water has very High specific heat �Specific � heat is measured in cal/ml/o. C or cal/g/ o. C

Specific Heat �High specific heat warms up slower AND �Cools off slower � http: //oceanservice. noaa. gov/education/pd/oceans_weather_climate/media/specific_heat. swf �More massive �http: //mw 2. concord. org/public/part 2/heat/p age 2. cml

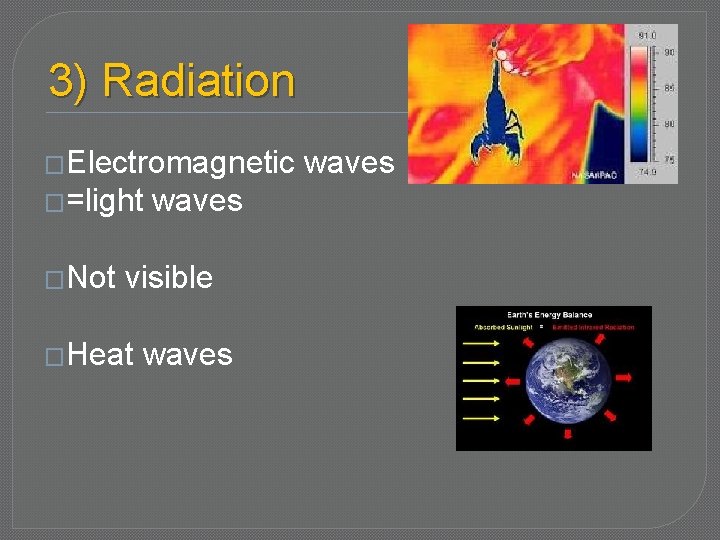
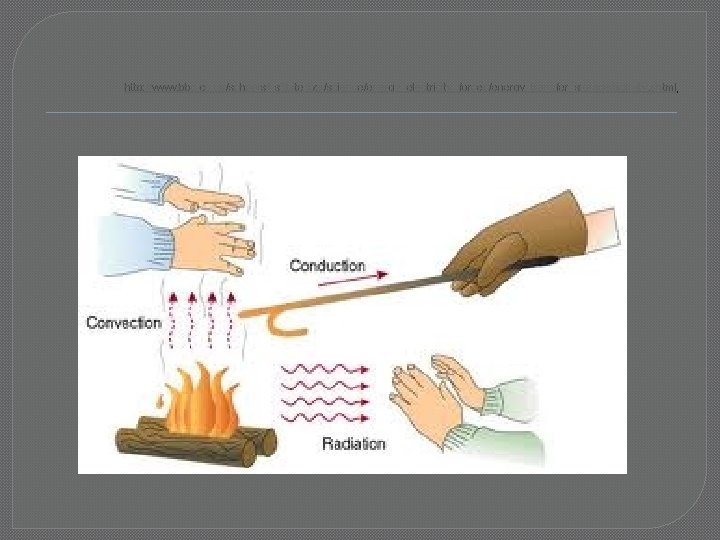
Heat is Transferred in 3 ways � 1) Conduction – objects touch, direct heat transfer from warm object to cold object � 2) Convection – heat transferred by flowing molecules � 3) Radiation – electromagnetic waves = � Infrared light waves � heat waves, not visible

1) Conduction �Metals � are good conductors because their electrons are loosely held (can flow) �Non-metals hold their electrons tightly so are poor conductors called… �insulators

�Good �cold � conductors at room temperature feel: to the touch because…. heat is leaving your hand quickly. �Poor conductors don’t feel as cold because heat is leaving more slowly

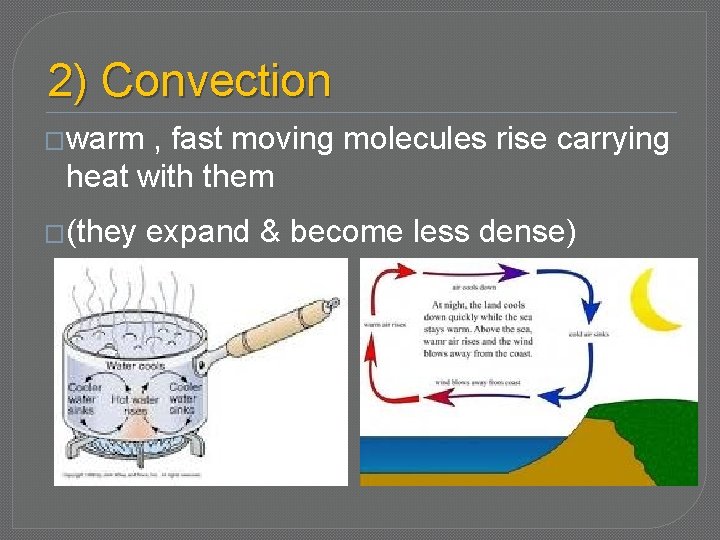
2) Convection �warm , fast moving molecules rise carrying heat with them �(they expand & become less dense)

3) Radiation �Electromagnetic �=light �Not waves visible �Heat waves

http: //www. bbc. co. uk/schools/ks 3 bitesize/science/energy_electricity_forces/energy_transfer_storage/activity. shtml

Heat transfer causes phase change �Lowest � � � energy = solid a) strongest intermolecular forces b) molecules vibrate in place so definite shape and volume �Medium � � � energy = Liquid a) weaker intermolecular forces allow molecules to flow around each other so no definite shape b) still to low energy for molecules to break away completely and expand so definite volume

�Highest � � � energy = gas a) weakest intermolecular forces b) molecules can flow & expand c) no definite shape or volume

Phase changes : �Change in amount of thermal energy cause molecules to move in different ways �Higher � � �Mass energy = weaker intermolecular forces as molecules expand does not change �Density changes

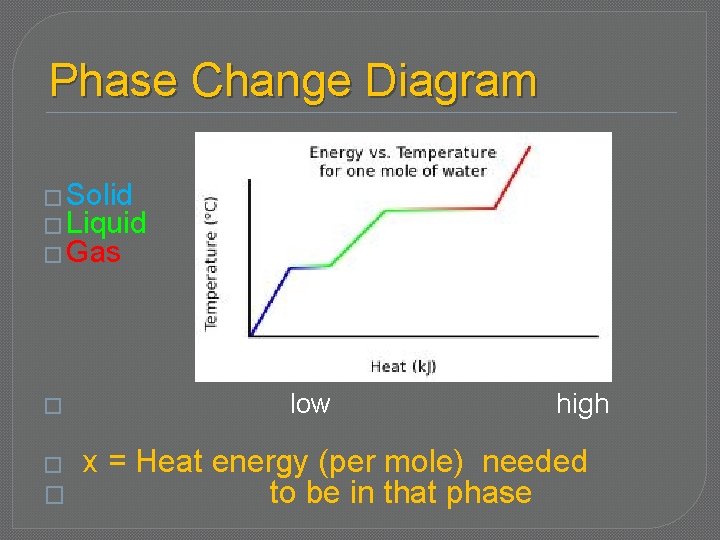
Phase Change Diagram � Solid � Liquid � Gas � � � low high x = Heat energy (per mole) needed to be in that phase

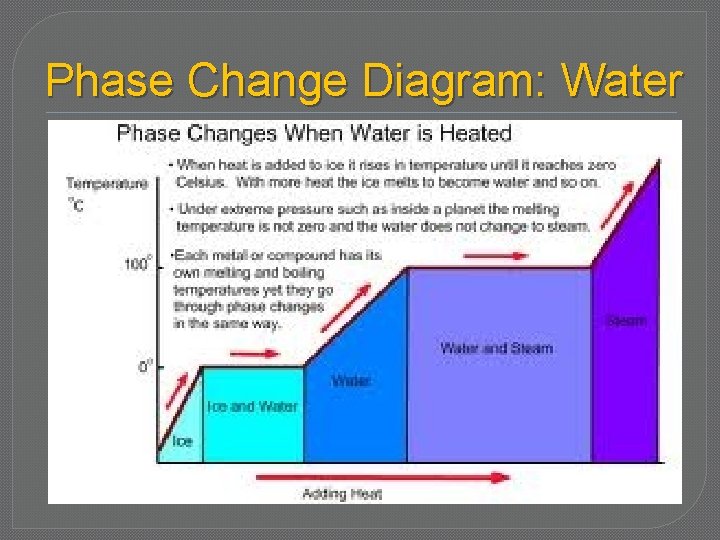
Phase Change Diagram: Water

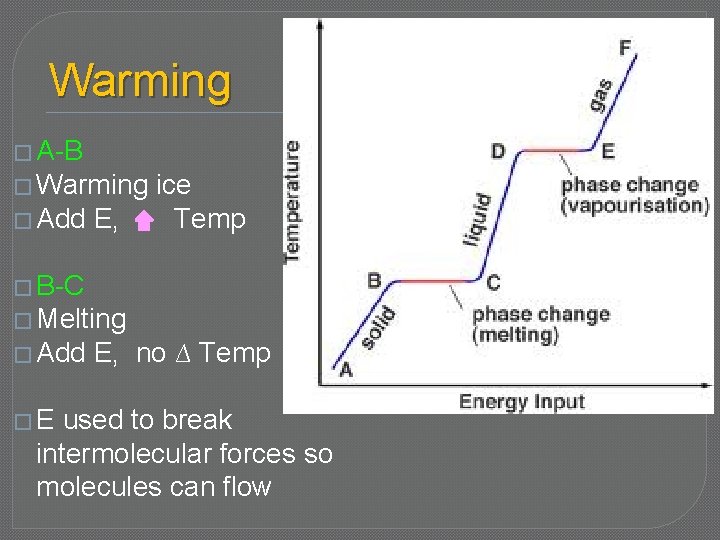
Warming � A-B � Warming � Add E, ice Temp � B-C � Melting � Add �E E, no ∆ Temp used to break intermolecular forces so molecules can flow

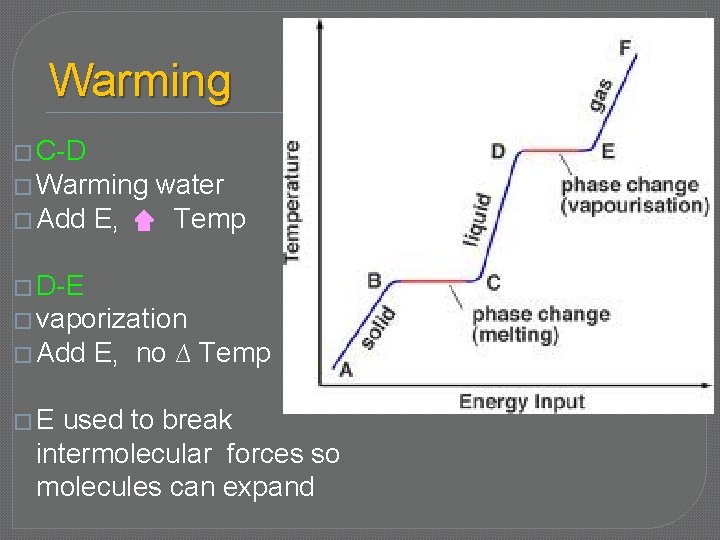
Warming � C-D � Warming � Add E, water Temp � D-E � vaporization � Add �E E, no ∆ Temp used to break intermolecular forces so molecules can expand

Vaporization changes liquid to gas � Boiling � Evaporation � Bubbles � High of gas form at bottom of liquid energy molecules break loose from surface of fluid

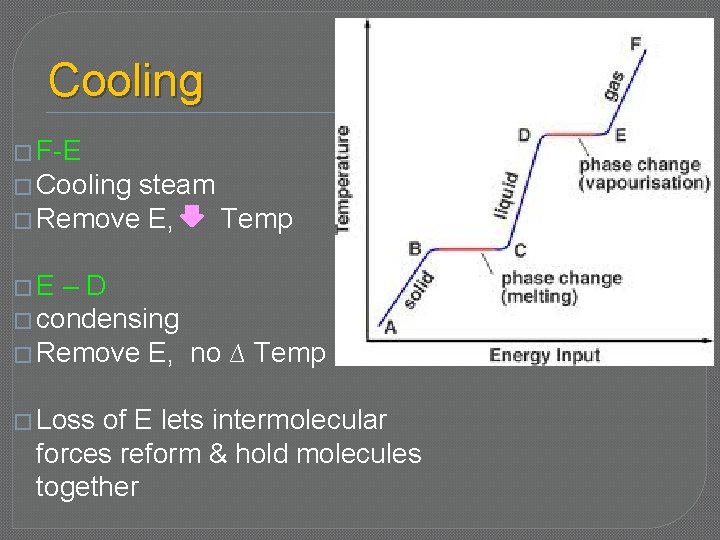
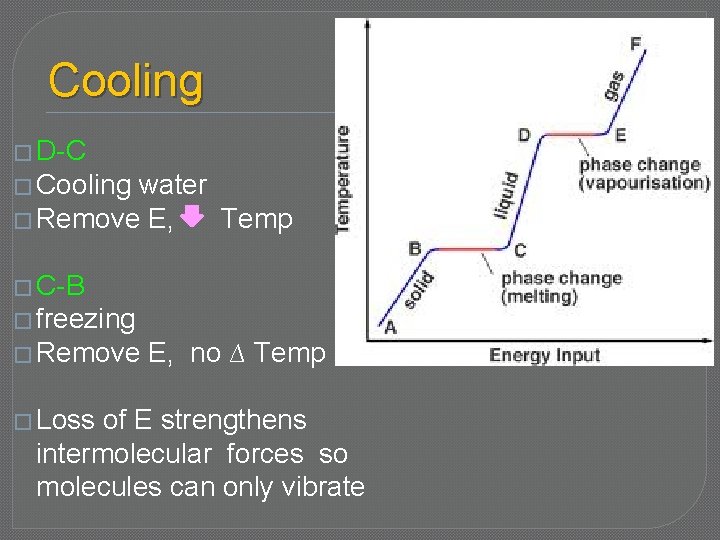
Cooling � F-E � Cooling steam � Remove E, Temp �E –D � condensing � Remove E, no ∆ Temp � Loss of E lets intermolecular forces reform & hold molecules together

Cooling � D-C � Cooling water � Remove E, Temp � C-B � freezing � Remove � Loss E, no ∆ Temp of E strengthens intermolecular forces so molecules can only vibrate