Heat Temperature And Phase Changes Pisgah High School













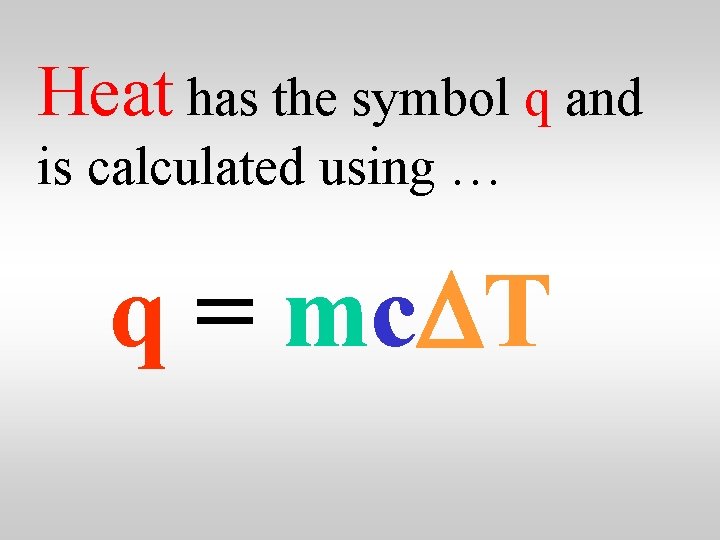
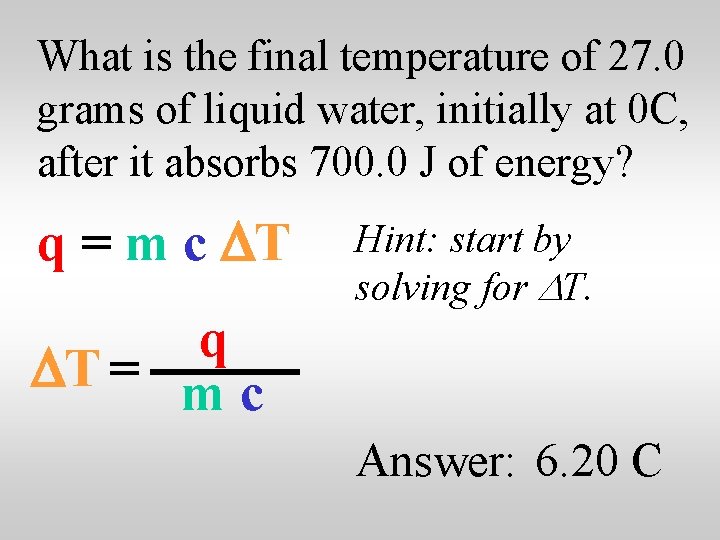































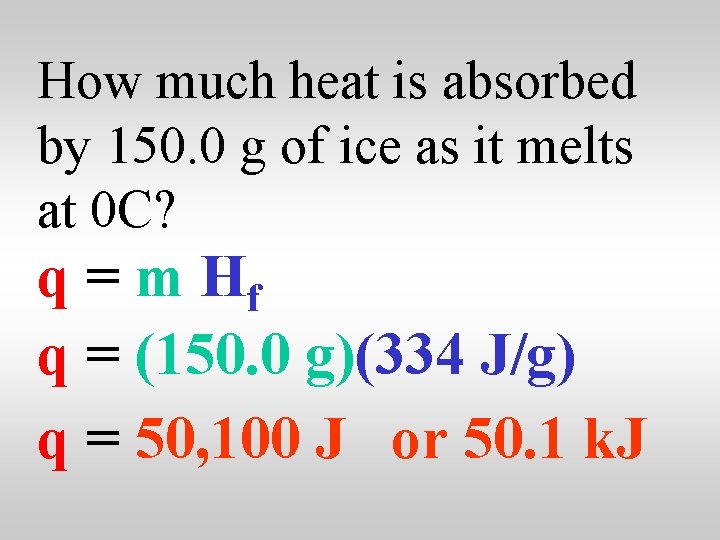

















- Slides: 160

Heat, Temperature And Phase Changes Pisgah High School Chemistry Mr. Jones Last rev. 5/12/04

Part One Heat and Temperature

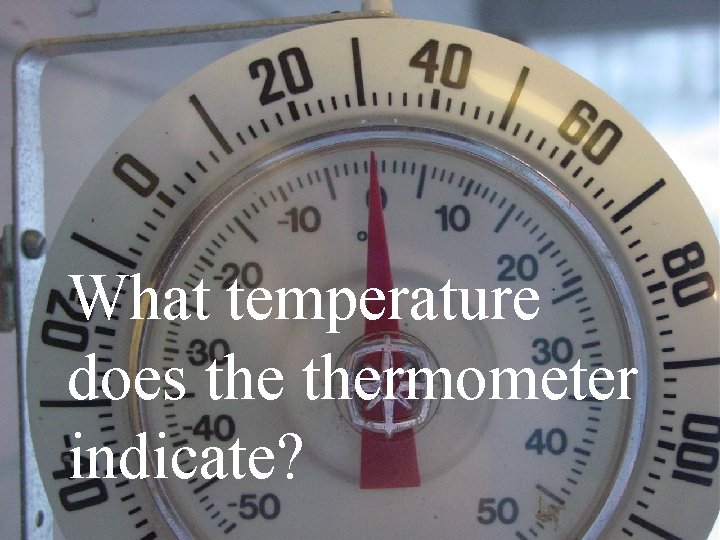
What temperature does thermometer indicate?

What might be going on that would cause this temperature?

This is the view out the window, past thermometer.

Yep. It is snowing.

Why would snow cause the air temperature to be at precisely 0 C? What occurs at 0 C? Water freezes and ice melts.

Ice, in the form of snow, falls through the slightly warmer air. The snow melts and absorbs heat from the air, causing the air to cool. Ice melts at 0 C, so the air cools to that temperature.

The temperature hovers at zero Celsius as the snow melts.

So why is there snow on the ground if it is melting? Yep. That’s what allows the snow to accumulate.

As the snow melts, it absorbs heat and cools the ground, the car, and the grill.

This allows more snow to lay. It doesn’t melt because the ground is now at 0 C.

What does it mean to have a temperature of 0 C? What is temperature? Is temperature the same thing as heat?

Temperature is a measure of how “hot” or “cold” something is. Temperature is measured in arbitrary units, like Fahrenheit or Celsius.

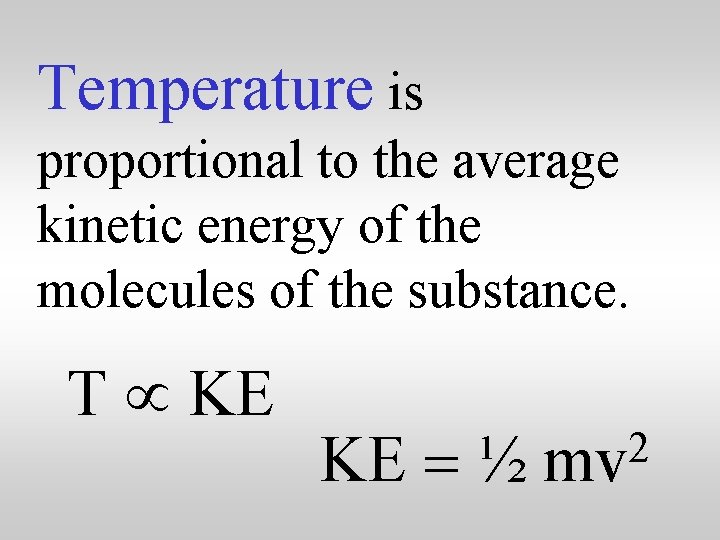
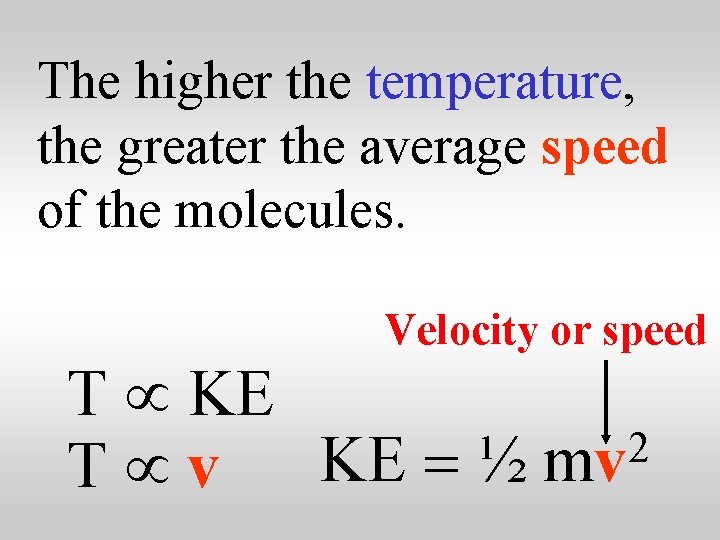
Temperature is proportional to the average kinetic energy of the molecules of the substance. T µ KE KE = ½ 2 mv

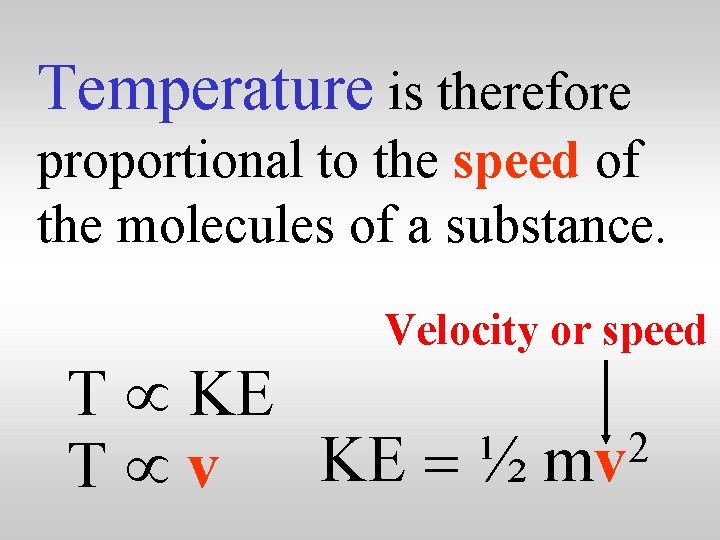
Temperature is therefore proportional to the speed of the molecules of a substance. Velocity or speed T µ KE 2 T µ v KE = ½ mv

The higher the temperature, the greater the average speed of the molecules. Velocity or speed T µ KE 2 T µ v KE = ½ mv

Heat is thermal energy transferred from a hot object to a cold object. Heat is measured in energy units -- Joules or calories.

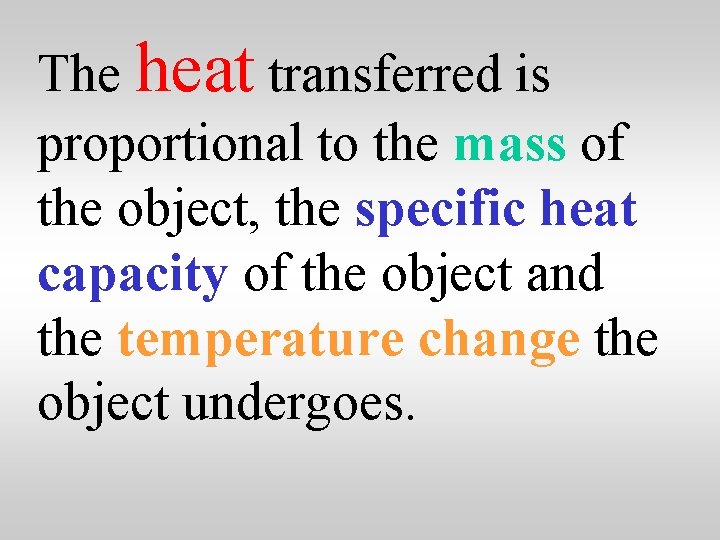
The heat transferred is proportional to the mass of the object, the specific heat capacity of the object and the temperature change the object undergoes.
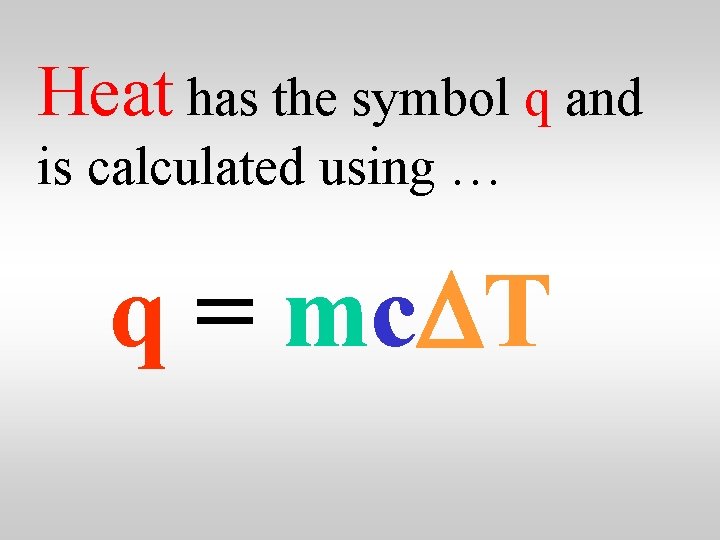
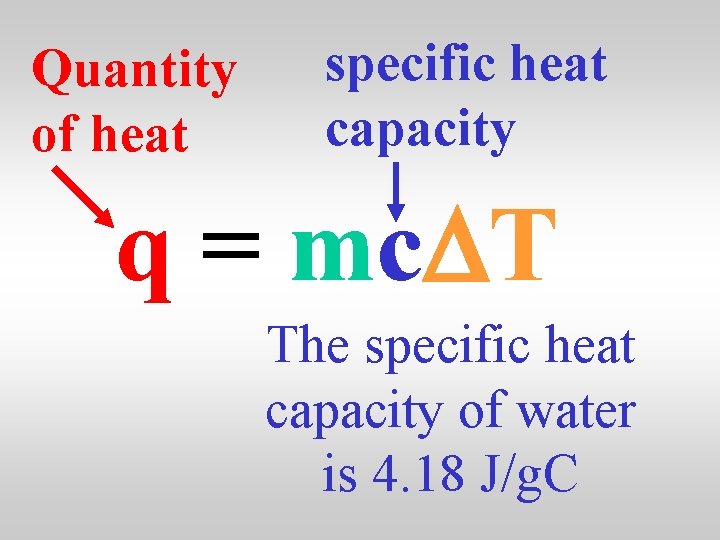
Heat has the symbol q and is calculated using … q = mc. DT

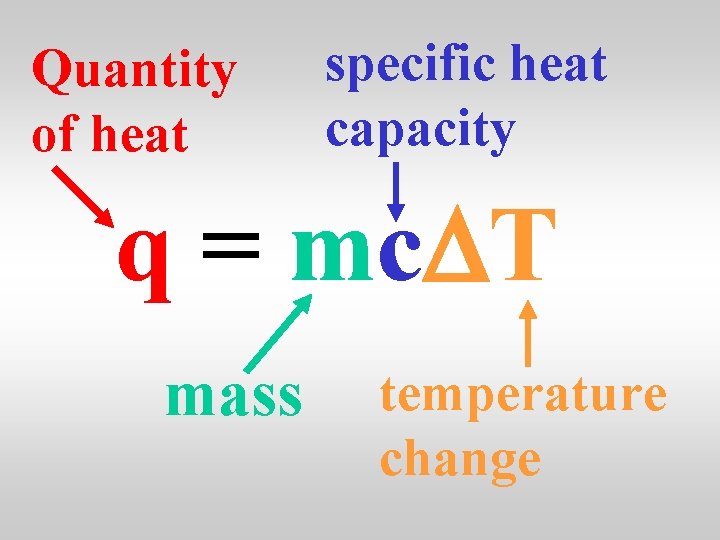
Quantity of heat specific heat capacity q = mc. DT mass temperature change

Quantity of heat specific heat capacity q = mc. DT The specific heat capacity of water is 4. 18 J/g. C

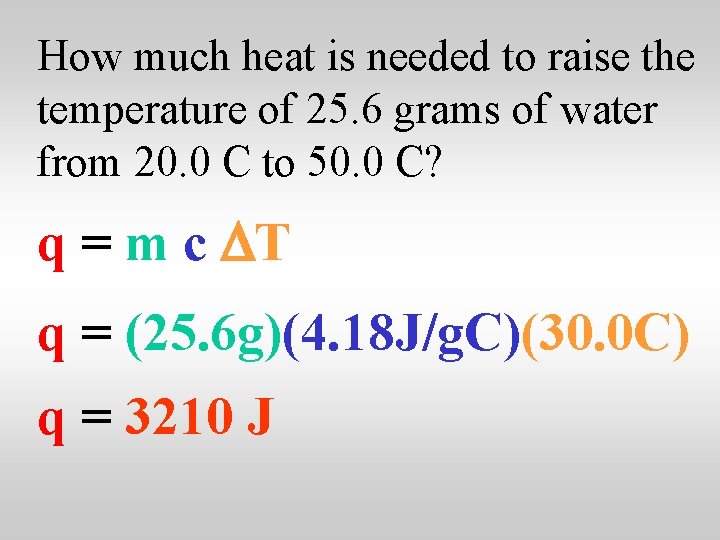
How much heat is needed to raise the temperature of 25. 6 grams of water from 20. 0 C to 50. 0 C? q = m c DT q = (25. 6 g)(4. 18 J/g. C)(30. 0 C) q = 3210 J
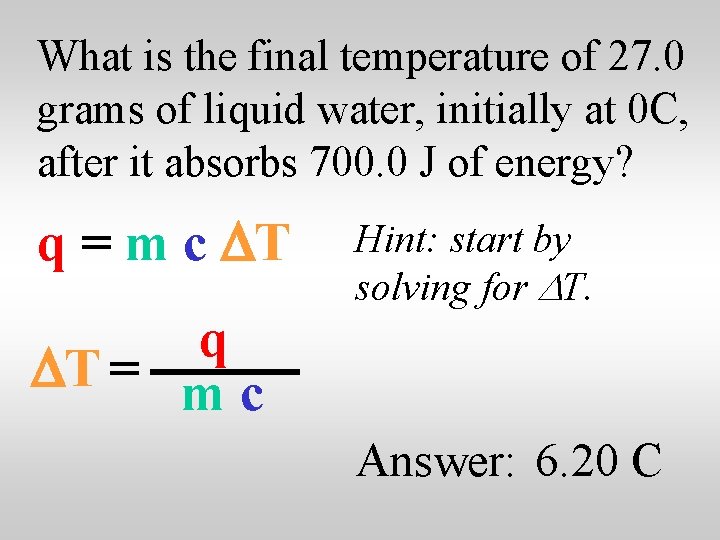
What is the final temperature of 27. 0 grams of liquid water, initially at 0 C, after it absorbs 700. 0 J of energy? q = m c DT q DT = m c Hint: start by solving for DT. Answer: 6. 20 C

Part Two Calorimetry and Specific Heat Capacity

Calorimetry is a collection of laboratory procedures used to investigate the transfer of heat. In calorimetry experiments, one might be looking for a final temperature or a specific heat capacity.

Investigate: Suppose two different masses of water at different temperatures are mixed. Can you predict the final temperature?

Investigate: Will the final temperature be cooler than the cool water, or will it be warmer than the warm water? Or will the final temperature be somewhere in between?

Investigate: Develop a procedure where you could mix a known mass of cool water with a different mass of water at an elevated temperature and measure the final (equilibrium) temperature. What equipment would you need?

Investigate: Develop a procedure where you could mix a known mass of cool water with a different mass of water at an elevated temperature and measure the final (equilibrium) temperature. You could use a balance, a thermometer, a coffee cup calorimeter, and a hot plate.

Investigate: What do you need in a data table? Mass of calorimeter cup Mass of cool water Initial temperature of cool water Mass of warm water Initial temperature of hot water Final temperature after mixing Feel free to make additions.

Investigate: Whenever we design an experiment we make some assumptions. Here a couple, can you add any more? The calorimeter cup is a perfect insulator and no heat is exchanged with the surroundings. Note: Hot plates and boiling water can cause severe burns.

Investigate: You might need a hint about how to calculate the results. What is the law of conservation of energy? Energy is neither created nor destroyed, only changed in form.

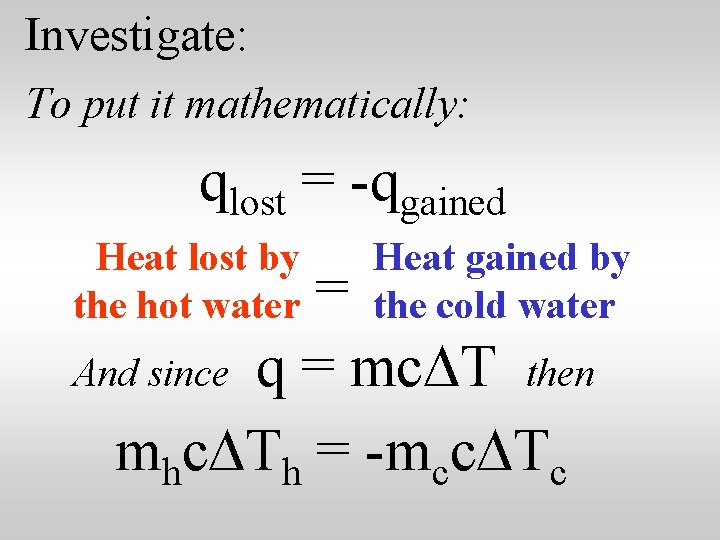
Investigate: You might need a hint about how to calculate the results. The law of conservation of energy suggests that the heat lost by the hot water as it cools is equal to the heat gained by the cool water as it warms up.

Investigate: To put it mathematically: qlost = -qgained Heat lost by the hot water Heat gained by the cold water = And since q = mc. DT then mhc. DTh = -mcc. DTc

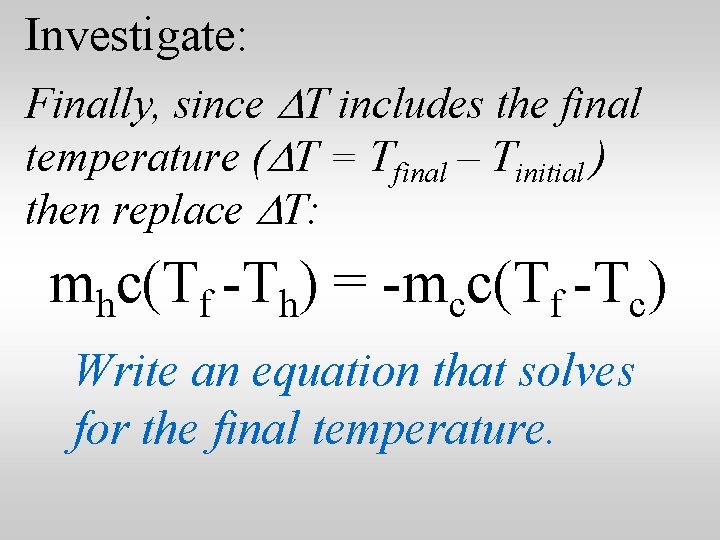
Investigate: Finally, since DT includes the final temperature (DT = Tfinal – Tinitial ) then replace DT: mhc(Tf -Th) = -mcc(Tf -Tc) Write an equation that solves for the final temperature.

Investigate: Use your equation to solve the following problem: Calculate the final temperature when 20. 0 grams of water at 85 C is added to 35. 0 grams of water at 10. 0 C in an insulated container.

Investigate: The answer to the following problem is 37. 3 C. Calculate the final temperature when 20. 0 grams of water at 85 C is added to 35. 0 grams of water at 10. 0 C in an insulated container.

In the next investigation you will … develop a method to find the specific heat capacity of a metal.

Specific heat capacity … 1. …varies from one substance to another 2. …a measure of how much heat something can “hold” 3. …the amount of heat needed to raise one gram of a substance by one Celsius degree

Specific heat capacity … 1. …varies from one substance to another 2. …a measure of how much heat something can “hold” 3. …the amount of heat needed to raise one gram of a substance by one Celsius degree

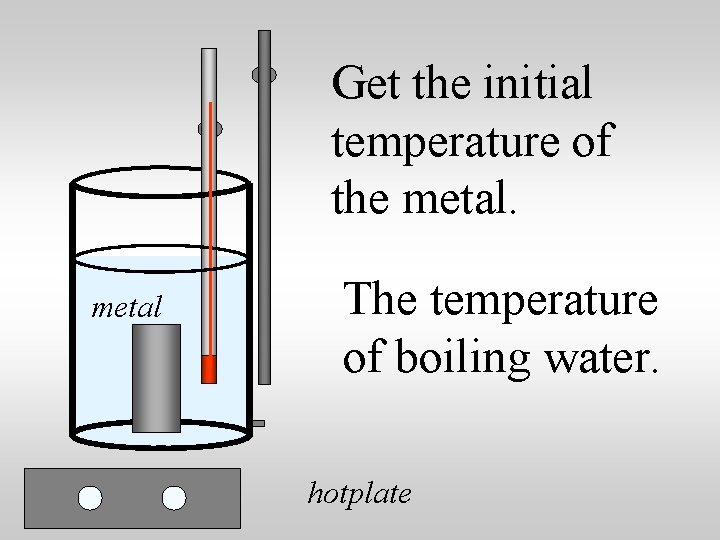
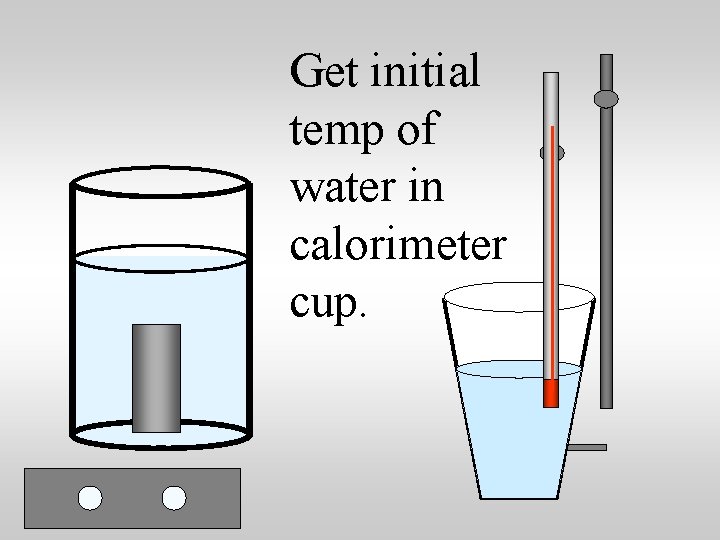
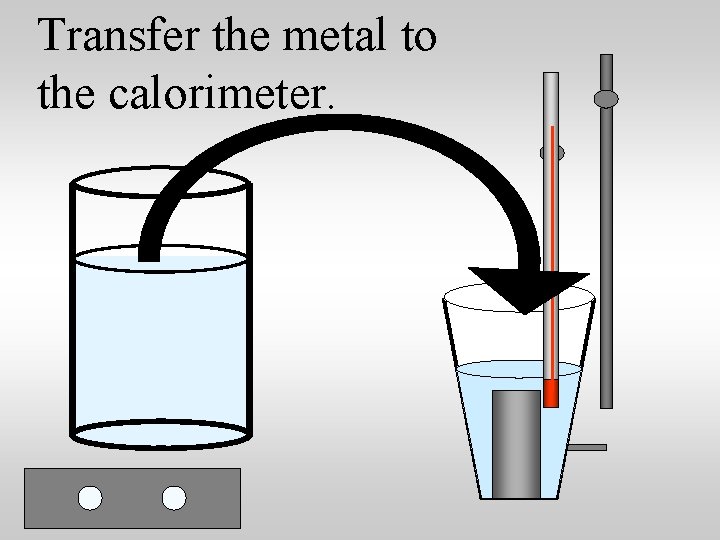
Specific heat capacity lab suggestions: 1. Heat a metal to a known temp 2. Transfer the metal to a known quantity of water at a known temperature 3. Measure the equilibrium temperature 4. Use qlost = qgained to compute the specific heat of the metal.

Get the initial temperature of the metal The temperature of boiling water. hotplate

Get initial temp of water in calorimeter cup.

Transfer the metal to the calorimeter.

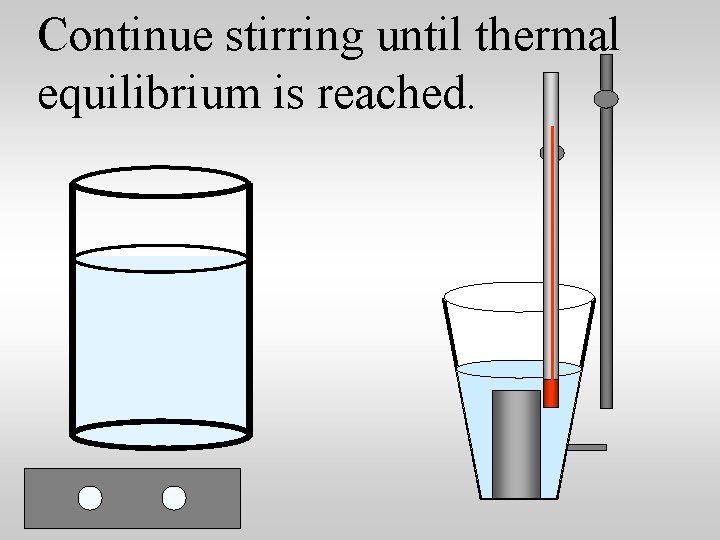
Continue stirring until thermal equilibrium is reached.

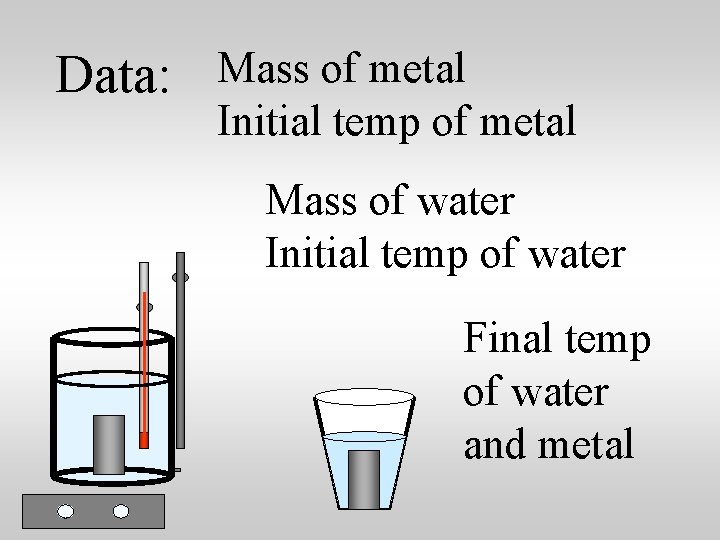
Data: Mass of metal Initial temp of metal Mass of water Initial temp of water Final temp of water and metal

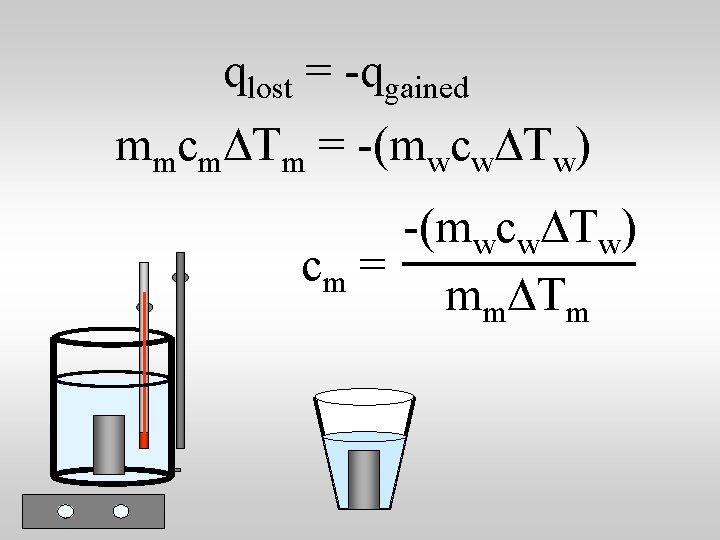
qlost = -qgained mmcm. DTm = -(mwcw. DTw) cm = mm. DTm

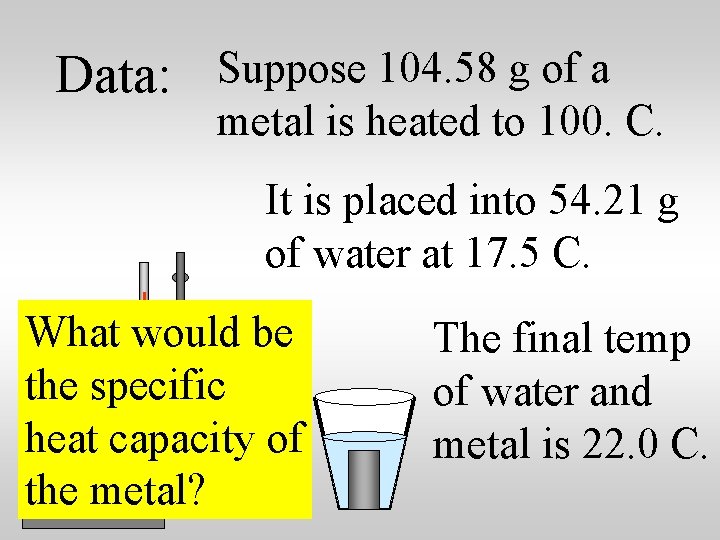
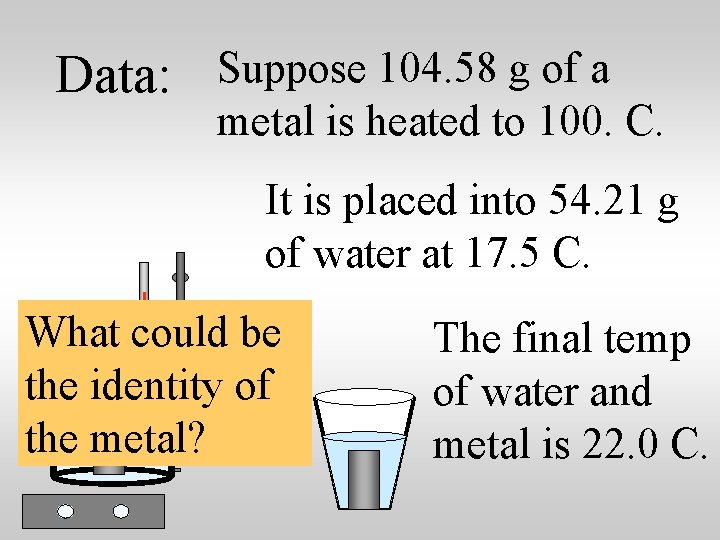
Data: Suppose 104. 58 g of a metal is heated to 100. C. It is placed into 54. 21 g of water at 17. 5 C. What would be the specific heat capacity of the metal? The final temp of water and metal is 22. 0 C.

Data: Suppose 104. 58 g of a metal is heated to 100. C. It is placed into 54. 21 g of water at 17. 5 C. What could be the identity of the metal? The final temp of water and metal is 22. 0 C.

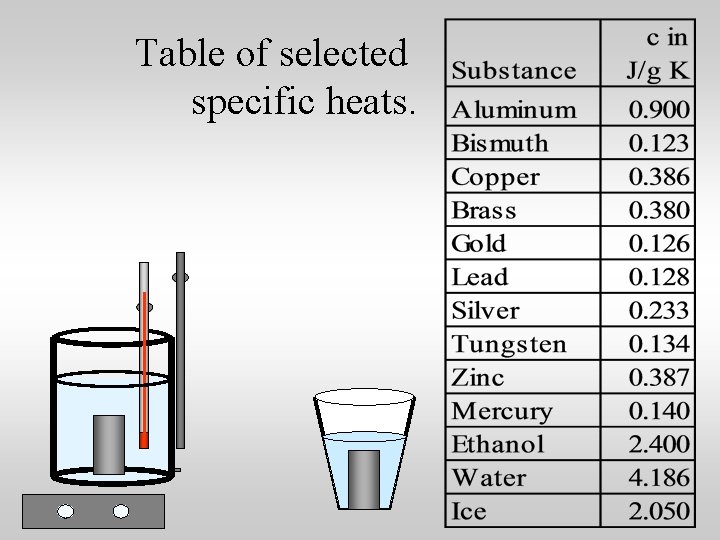
Table of selected specific heats.

Part Three Calorimetry and Phase Changes

Is heat is absorbed or released during a phase change? How could you measure the heat absorbed or released as substances change phase?

Consider ice melting in water. 1. Does the temperature of the water change? 2. Is the water absorbing or releasing heat? 3. Does ice absorb heat or release heat as it melts?

Consider ice melting in water. 1. Does the temperature of the water change? No 2. Is the water absorbing or releasing heat? Releasing heat 3. Does ice absorb heat or release heat as it melts? Absorb heat

Consider ice melting in water. The word fusion means “melting”. How could you design an experiment to measure the heat of fusion of ice?

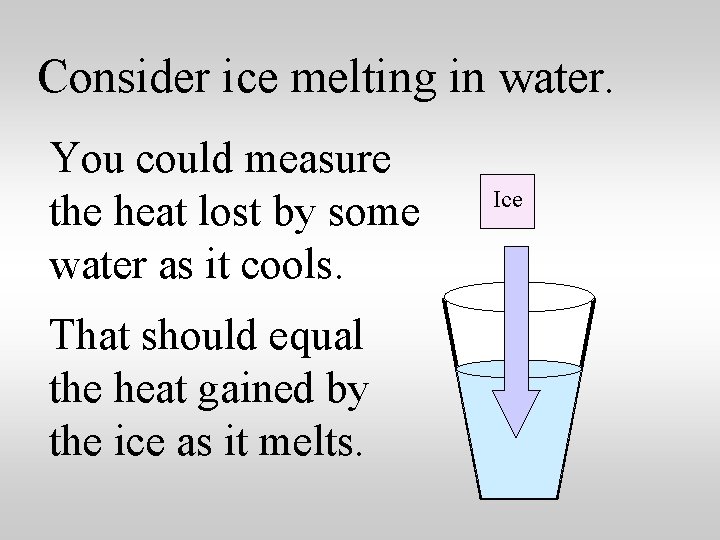
Consider ice melting in water. You could measure the heat lost by some water as it cools. That should equal the heat gained by the ice as it melts. Ice

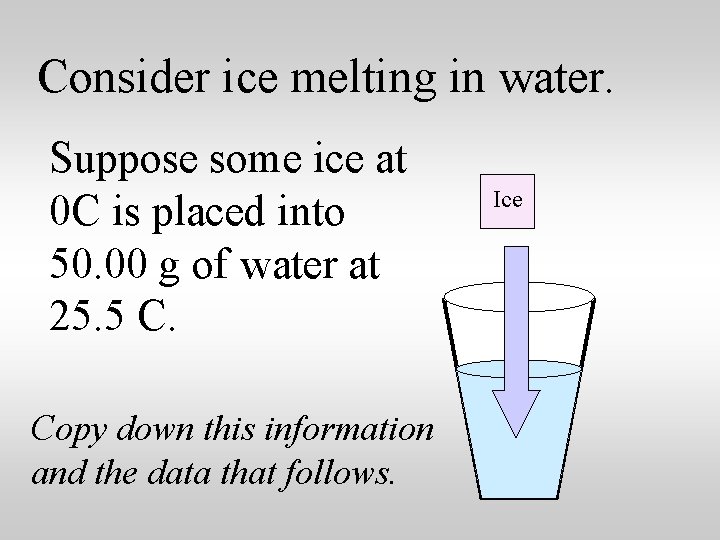
Consider ice melting in water. Suppose some ice at 0 C is placed into 50. 00 g of water at 25. 5 C. Copy down this information and the data that follows. Ice

Consider ice melting in water. When the system reaches equilibrium at 0 C, 15. 95 grams of the ice has melted. Ice

Consider ice melting in water. Knowing that the heat lost by the water as it cools to 0 C is equal to the heat gained by the ice as it melts at 0 C … Ice

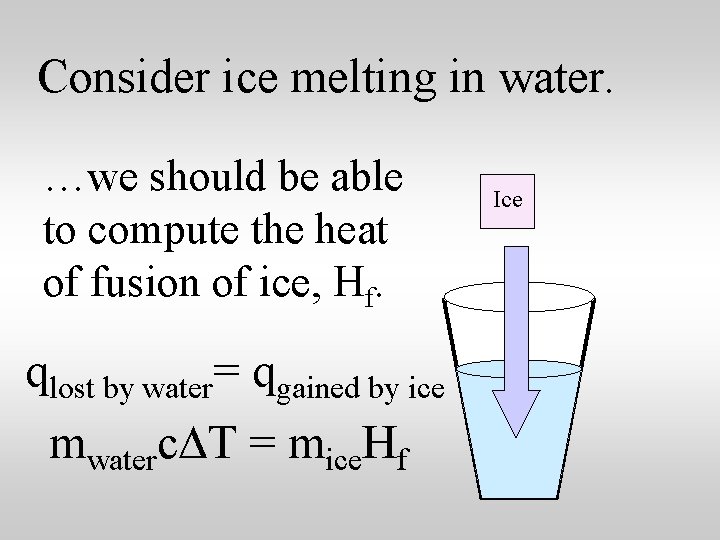
Consider ice melting in water. …we should be able to compute the heat of fusion of ice, Hf. qlost by water= qgained by ice mwaterc. DT = mice. Hf Ice

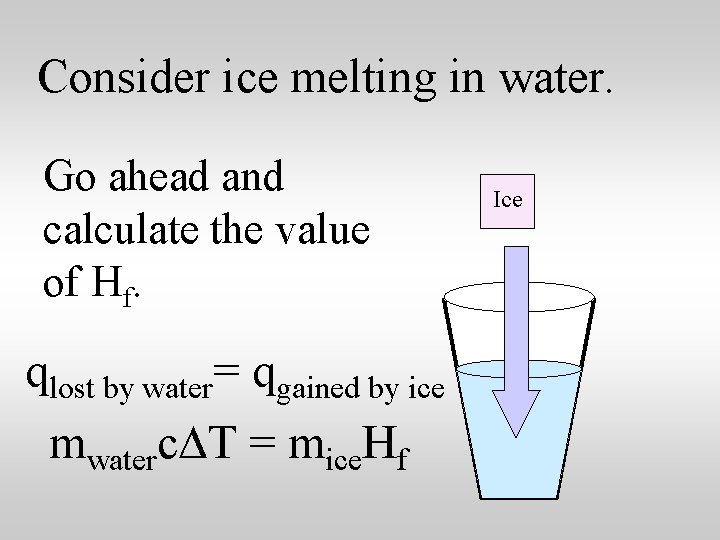
Consider ice melting in water. Go ahead and calculate the value of Hf. qlost by water= qgained by ice mwaterc. DT = mice. Hf Ice

We now know that heat is absorbed or released during a phase change. Heat is absorbed as solids melt, or liquids vaporize.

We now know that heat is absorbed or released during a phase change. Heat is released as liquids freeze, or vapors condense.

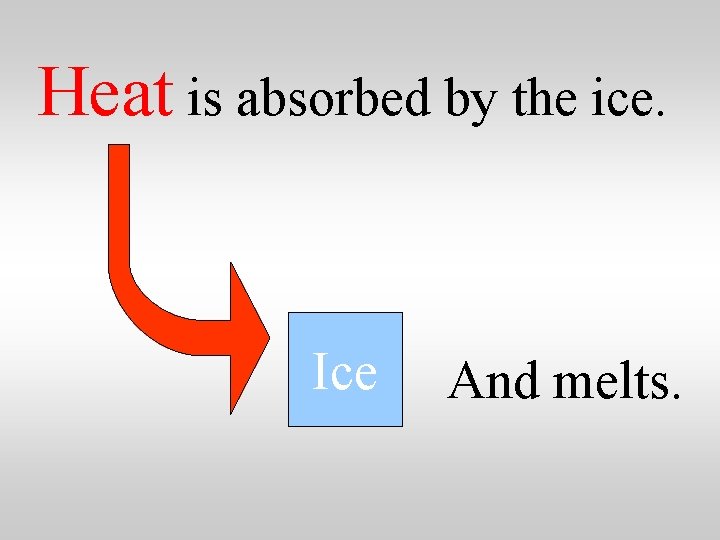
Heat is absorbed by the ice. Ice And melts.

Heat is absorbed by the ice. One gram of ice at 0 C absorbs 334 J as it melts to form water at 0 C. … making liquid water

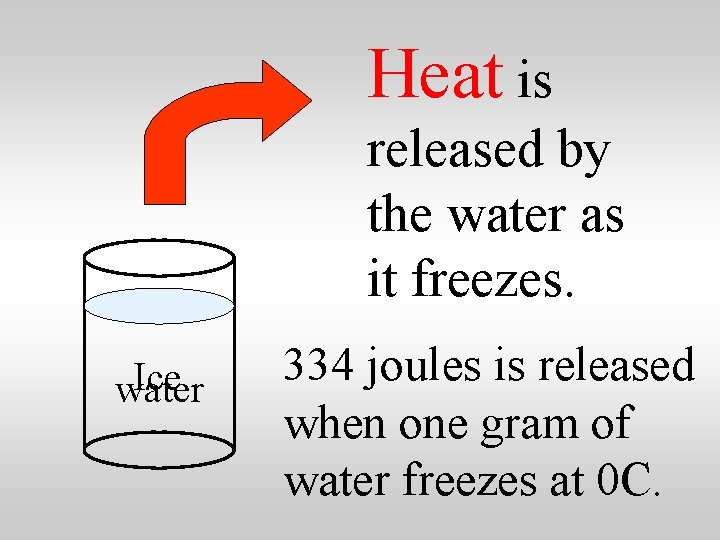
Heat is released by the water as it freezes. Ice water 334 joules is released when one gram of water freezes at 0 C.

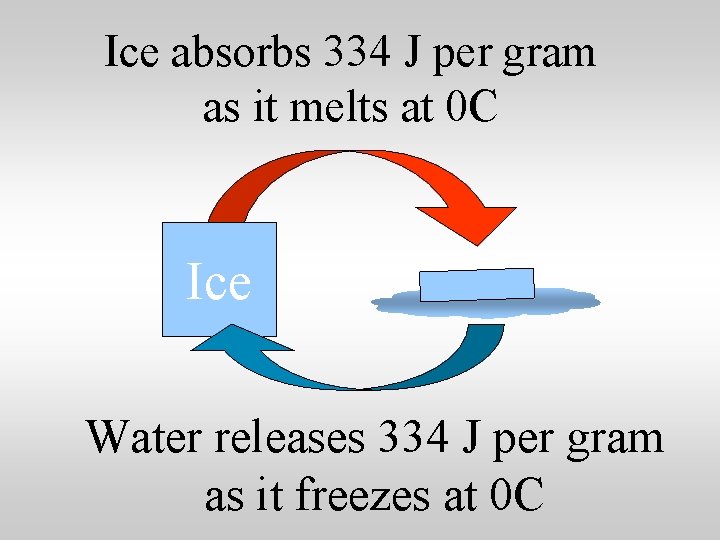
Ice absorbs 334 J per gram as it melts at 0 C Ice Water releases 334 J per gram as it freezes at 0 C

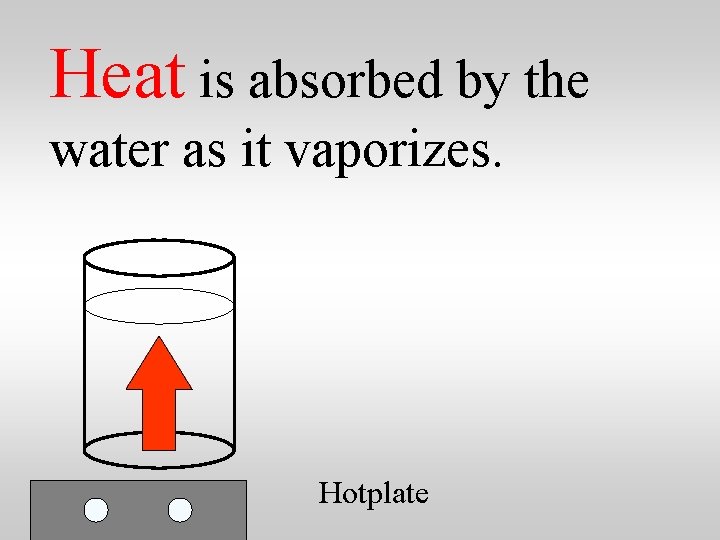
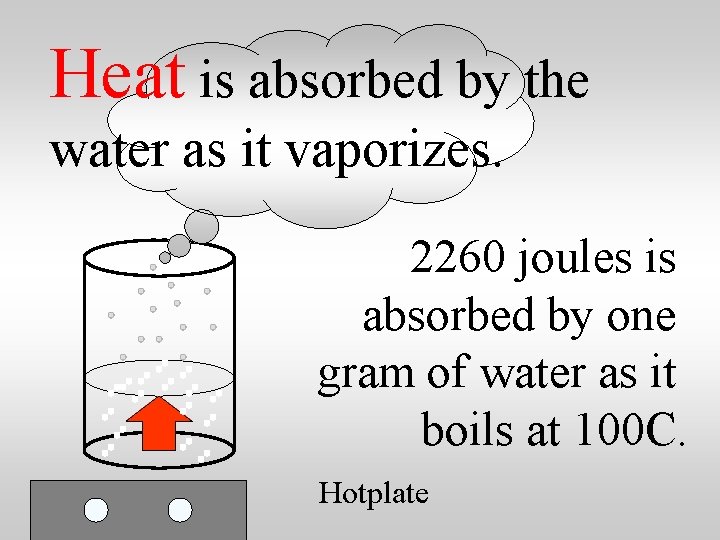
Heat is absorbed by the water as it vaporizes. Hotplate

Heat is absorbed by the water as it vaporizes. 2260 joules is absorbed by one gram of water as it boils at 100 C. Hotplate

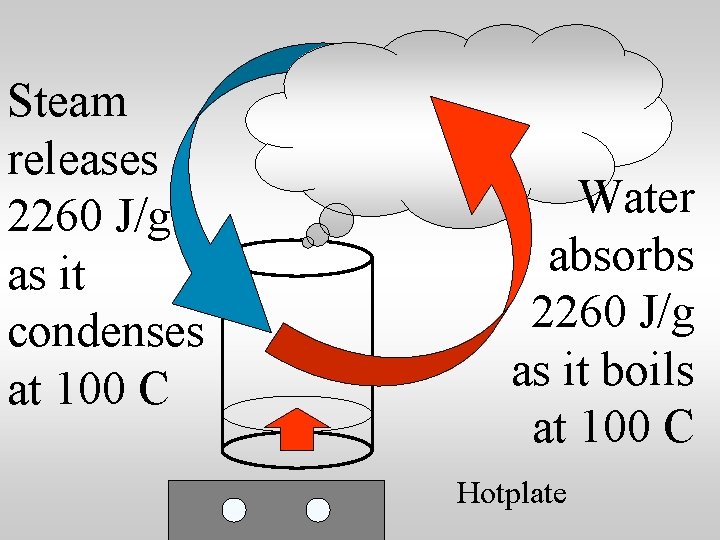
Steam releases 2260 J/g as it condenses at 100 C Water absorbs 2260 J/g as it boils at 100 C Hotplate

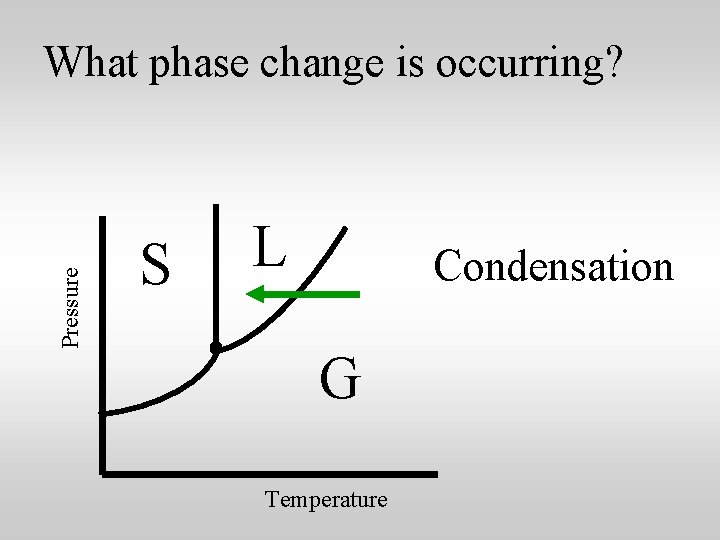
Heat is released by water vapor as it condenses.

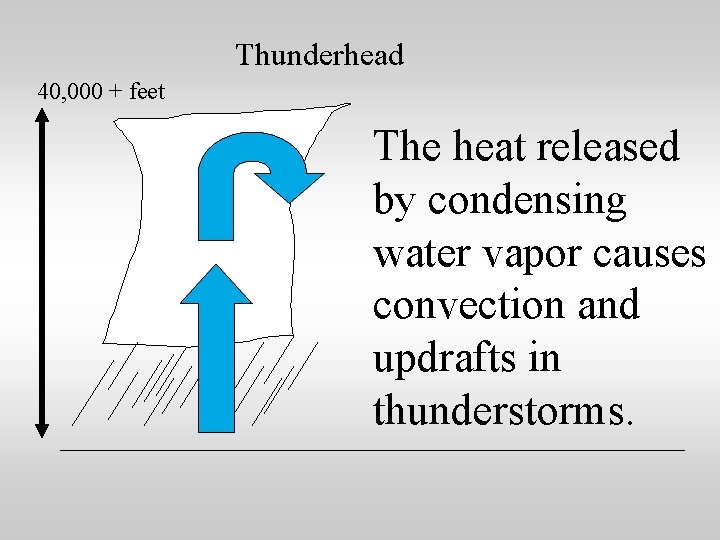
Heat is released by water vapor as it condenses. The heat released by condensing water vapor is a major factor in weather phenomena like thunderstorms and hurricanes.

Thunderhead 40, 000 + feet The heat released by condensing water vapor causes convection and updrafts in thunderstorms.

Phase changes occur at a constant temperature as heat is absorbed or released.

Question for discussion: If phase changes occur at a constant temperature, then what happens to the heat when water boils?

Question for discussion – possible answers: a. Heat energy is converted to matter (E=mc 2) and it stays in the water. b. The heat increases the speed of the water molecules. c. The heat energy breaks the intermolecular bonds which keep the water in the liquid phase. d. The temperature really does change, you just missed it.

Question for discussion – possible answers: a. Heat energy is converted to matter (E=mc 2) and it stays in the water. b. The heat increases the speed of the water molecules. c. The heat energy breaks the intermolecular bonds which keep the water in the liquid phase. d. The temperature really does change, you just missed it.

Question for discussion – possible answers: a. Heat energy is converted to matter (E=mc 2) and it stays in the water. b. The heat increases the speed of the water molecules. c. The heat energy breaks the intermolecular bonds which keep the water in the liquid phase. d. The temperature really does change, you just missed it.

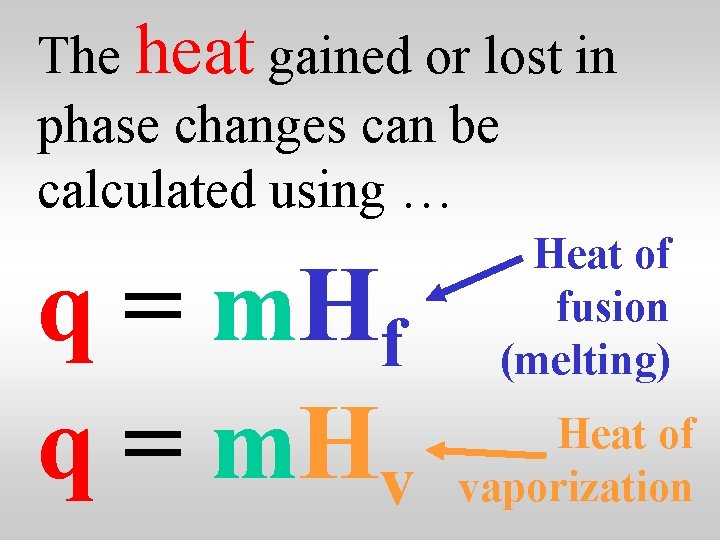
The heat gained or lost in phase changes can be calculated using … q = m. Hf q = m. Hv Heat of fusion (melting) Heat of vaporization

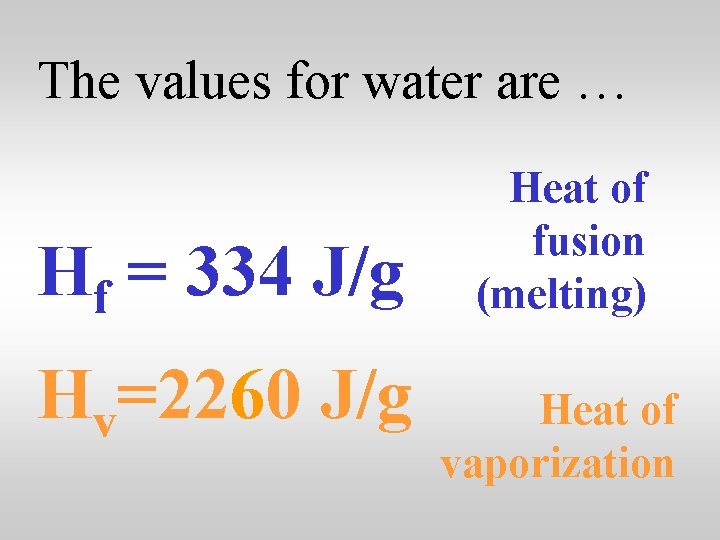
The values for water are … Hf = 334 J/g Hv=2260 J/g Heat of fusion (melting) Heat of vaporization
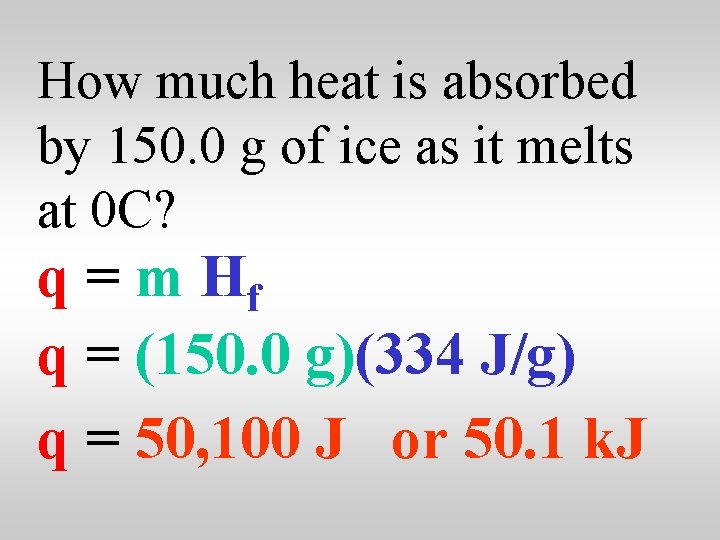
How much heat is absorbed by 150. 0 g of ice as it melts at 0 C? q = m Hf q = (150. 0 g)(334 J/g) q = 50, 100 J or 50. 1 k. J

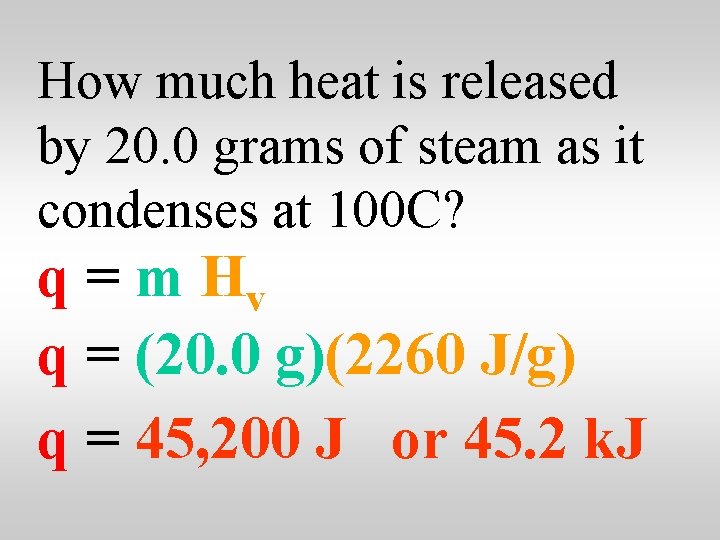
How much heat is released by 20. 0 grams of steam as it condenses at 100 C? q = m Hv q = (20. 0 g)(2260 J/g) q = 45, 200 J or 45. 2 k. J

Part Four Sublimation and Phase Diagrams

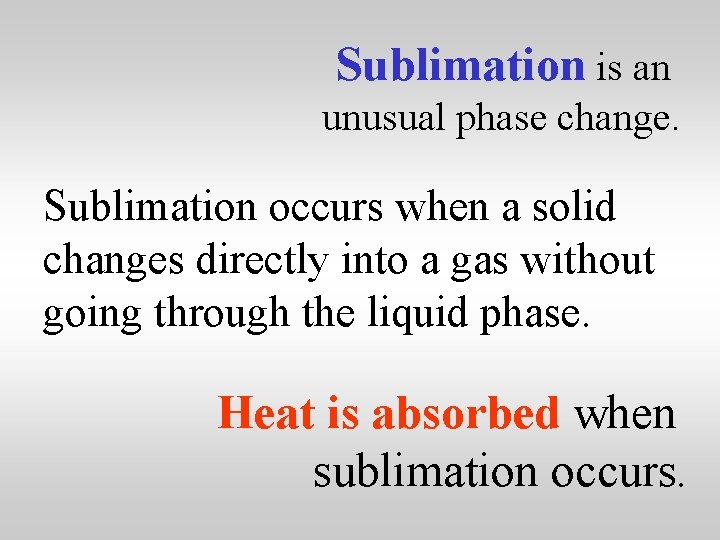
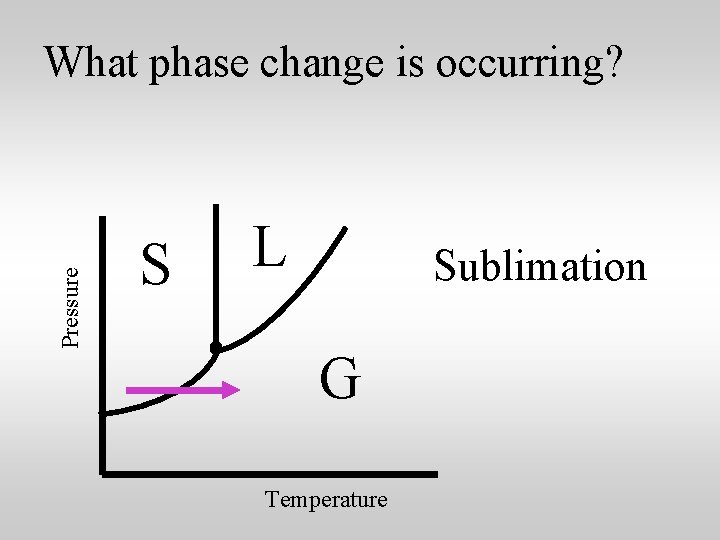
Sublimation is an unusual phase change. Sublimation occurs when a solid changes directly into a gas without going through the liquid phase. Heat is absorbed when sublimation occurs.

Dry ice is solid carbon dioxide, CO 2. At room temperature and normal atmospheric pressures dry ice undergoes sublimation. CO 2 vapor Dry Ice It goes directly from the solid state to the vapor state.

CO 2 vapor Dry ice is solid carbon dioxide, CO 2 vapor At room temperature and CO 2 vapor normal atmospheric pressures dry ice undergoes sublimation. CO 2 vapor Dry Ice It goes directly from the solid state to the vapor state.

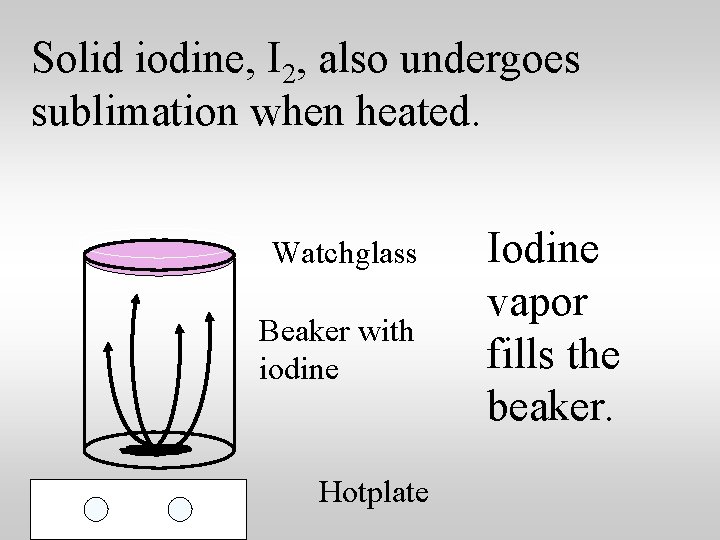
Solid iodine, I 2, also undergoes sublimation when heated. Watchglass Beaker with iodine Hotplate Iodine vapor fills the beaker.

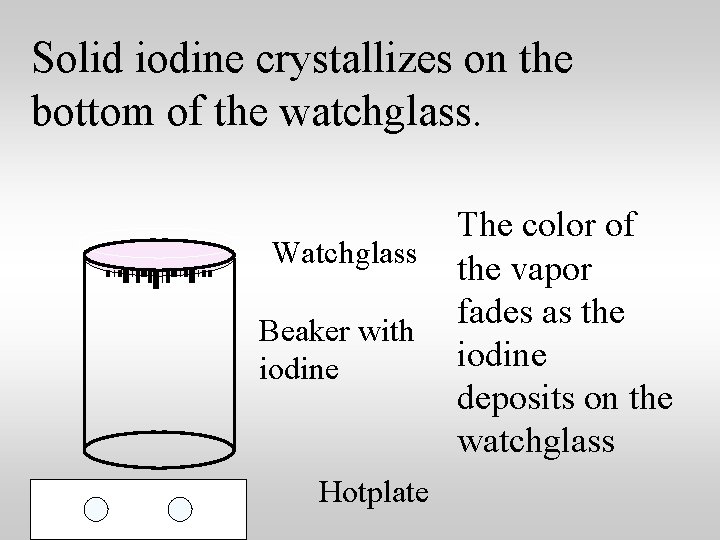
Solid iodine crystallizes on the bottom of the watchglass. Watchglass Beaker with iodine Hotplate The color of the vapor fades as the iodine deposits on the watchglass

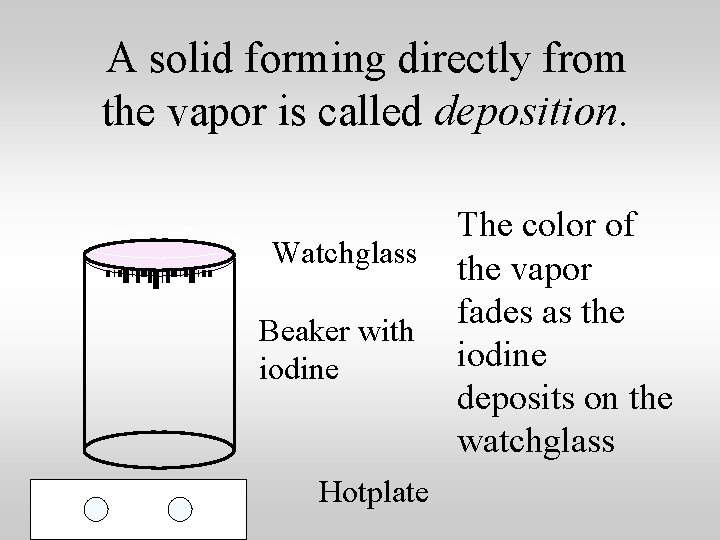
A solid forming directly from the vapor is called deposition. Watchglass Beaker with iodine Hotplate The color of the vapor fades as the iodine deposits on the watchglass

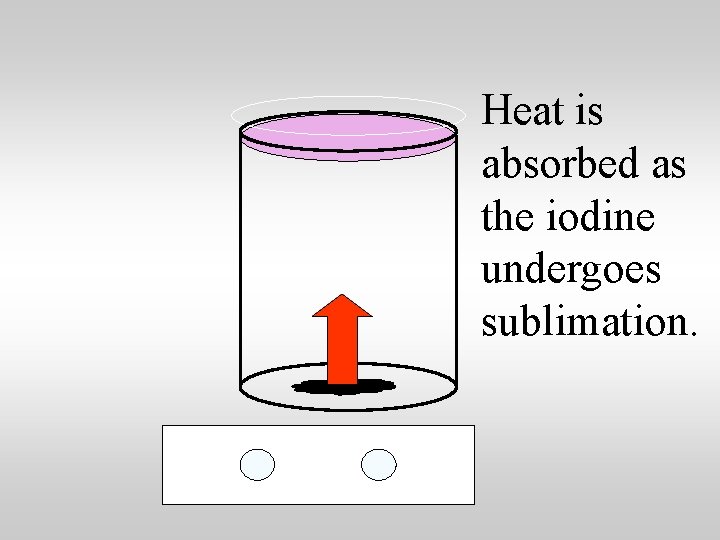
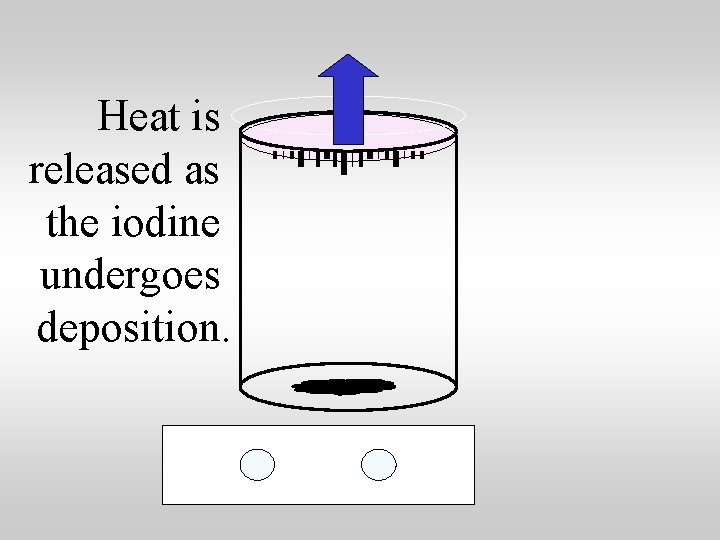
Heat is absorbed as the iodine undergoes sublimation.

Heat is released as the iodine undergoes deposition.

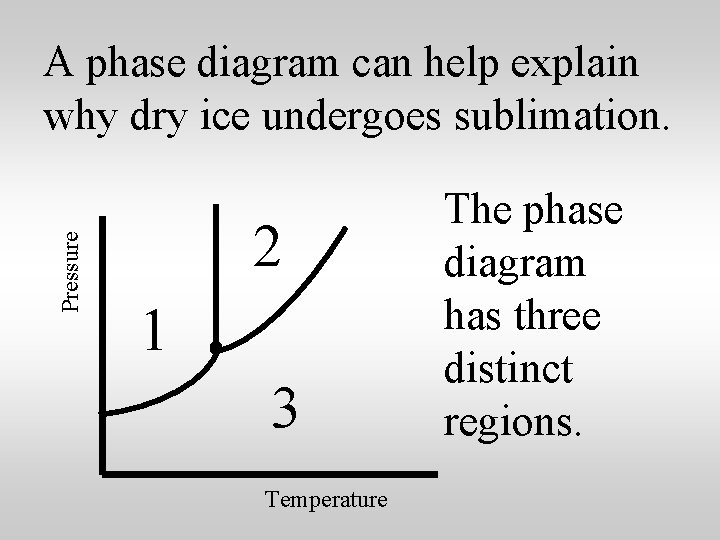
Pressure A phase diagram can help explain why dry ice undergoes sublimation. 2 1 3 Temperature The phase diagram has three distinct regions.

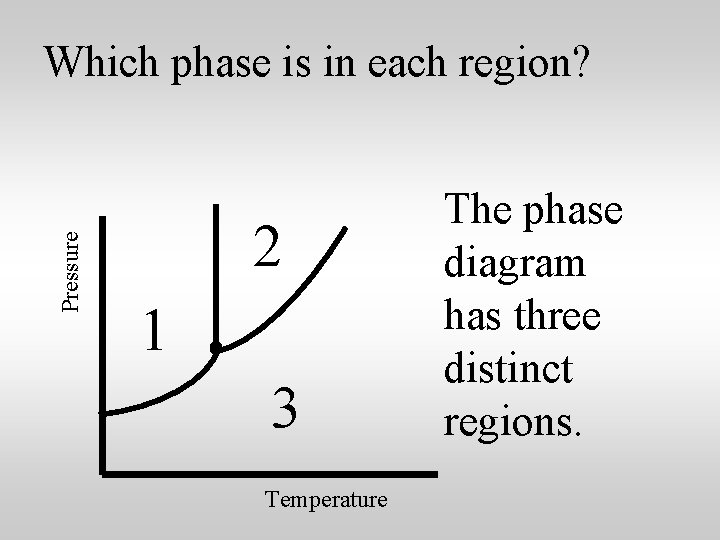
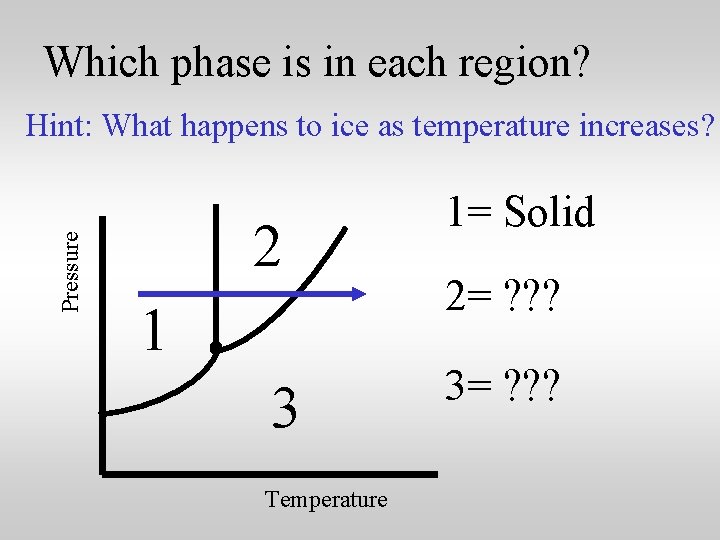
Pressure Which phase is in each region? 2 1 3 Temperature The phase diagram has three distinct regions.

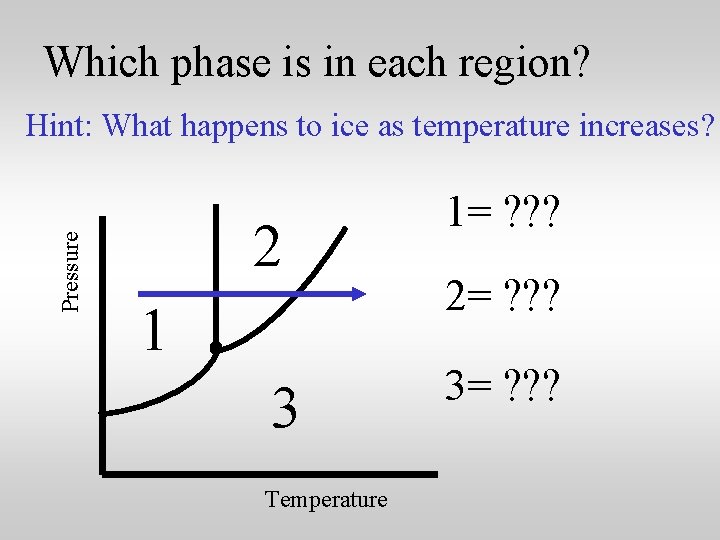
Which phase is in each region? Pressure Hint: What happens to ice as temperature increases? 2 1 3 Temperature 1= ? ? ? 2= ? ? ? 3= ? ? ?

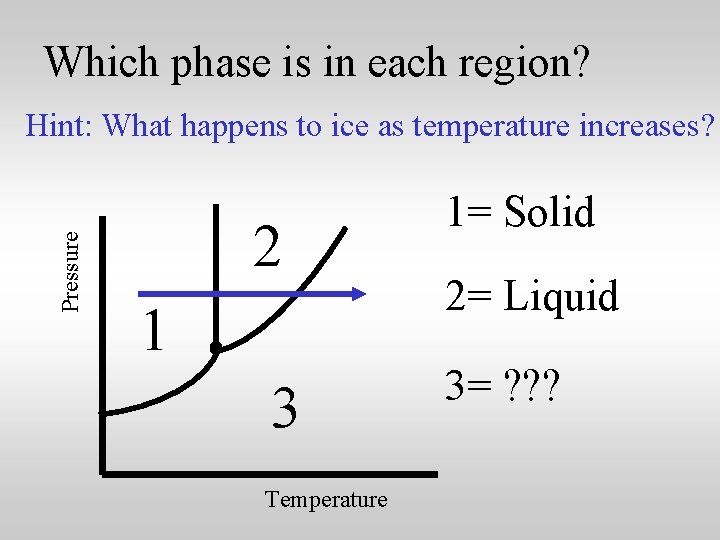
Which phase is in each region? Pressure Hint: What happens to ice as temperature increases? 2 1 3 Temperature 1= Solid 2= ? ? ? 3= ? ? ?

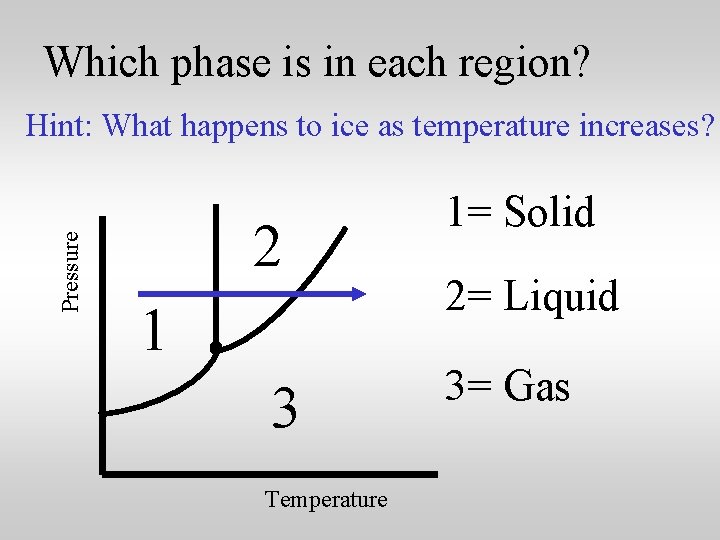
Which phase is in each region? Pressure Hint: What happens to ice as temperature increases? 2 1 3 Temperature 1= Solid 2= Liquid 3= ? ? ?

Which phase is in each region? Pressure Hint: What happens to ice as temperature increases? 2 1 3 Temperature 1= Solid 2= Liquid 3= Gas

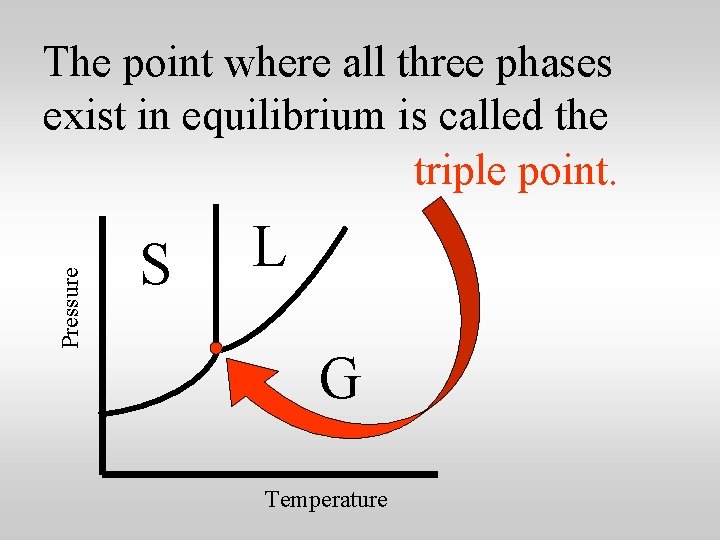
Pressure The point where all three phases exist in equilibrium is called the triple point. S L G Temperature

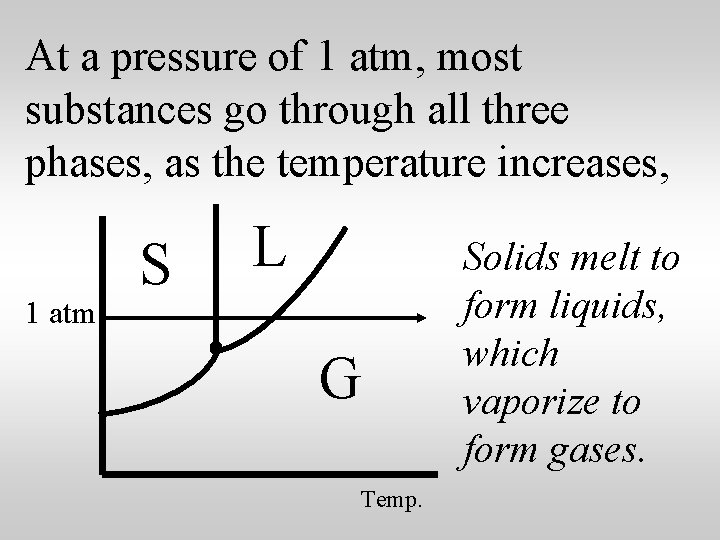
At a pressure of 1 atm, most substances go through all three phases, as the temperature increases, 1 atm S L G Temp. Solids melt to form liquids, which vaporize to form gases.

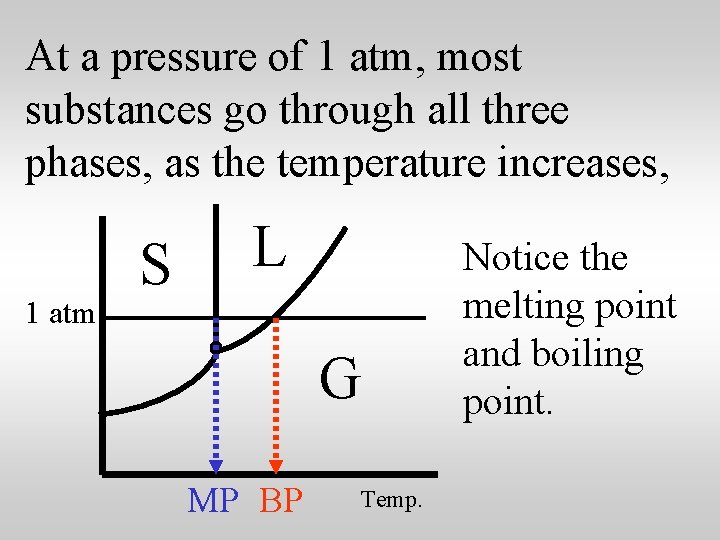
At a pressure of 1 atm, most substances go through all three phases, as the temperature increases, 1 atm S L G MP BP Temp. Notice the melting point and boiling point.

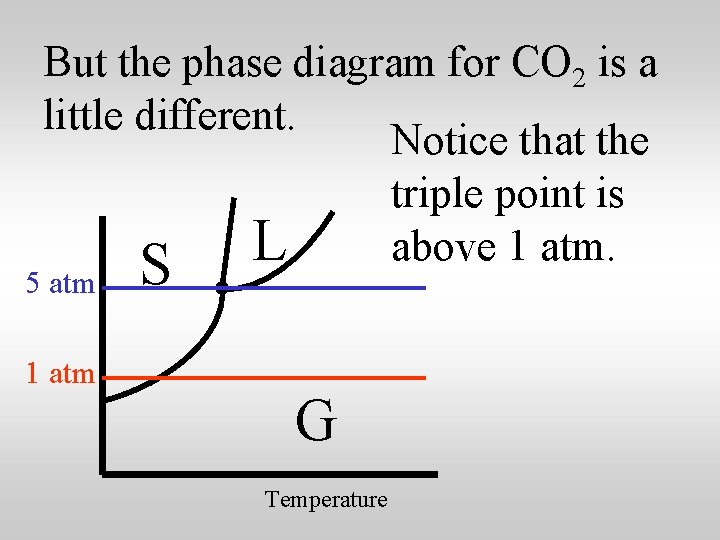
But the phase diagram for CO 2 is a little different. Notice that the triple point is L above 1 atm. 5 atm 1 atm S G Temperature

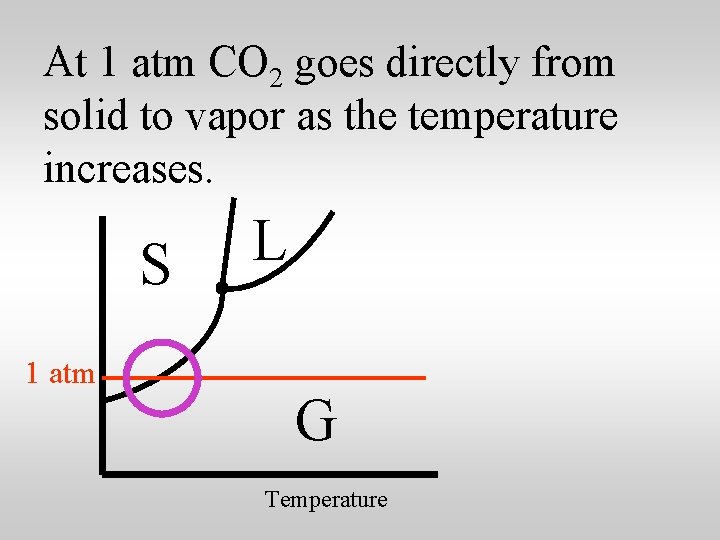
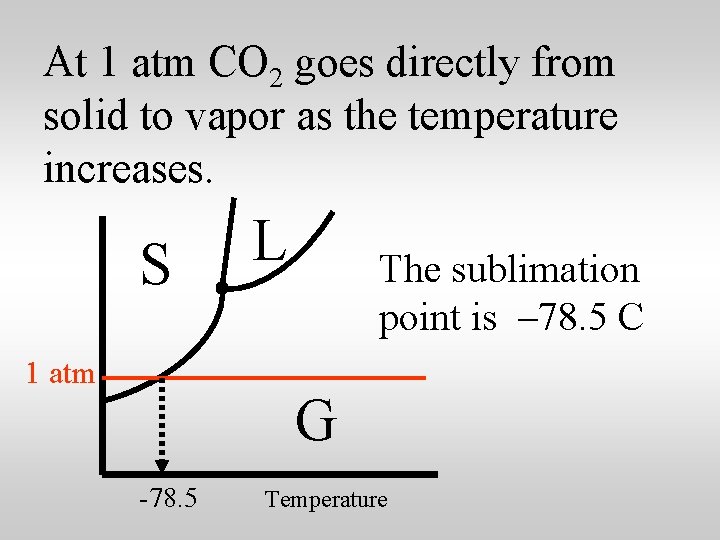
At 1 atm CO 2 goes directly from solid to vapor as the temperature increases. S 1 atm L G Temperature

At 1 atm CO 2 goes directly from solid to vapor as the temperature increases. S 1 atm L The sublimation point is – 78. 5 C G -78. 5 Temperature

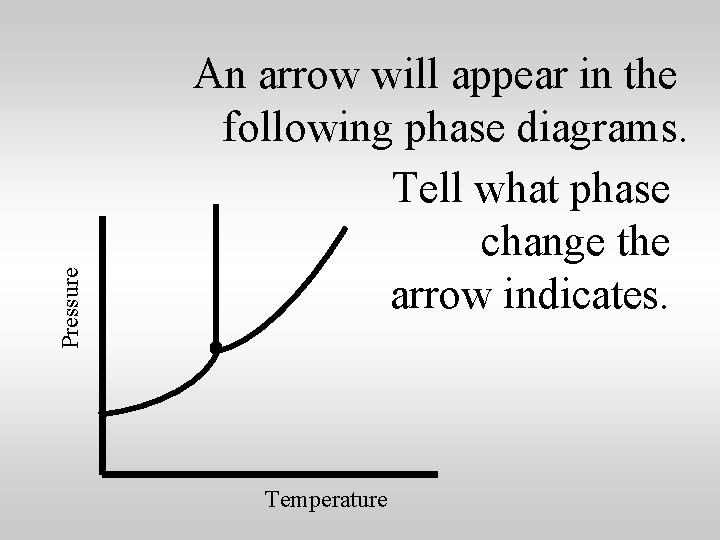
Pressure An arrow will appear in the following phase diagrams. Tell what phase change the arrow indicates. Temperature

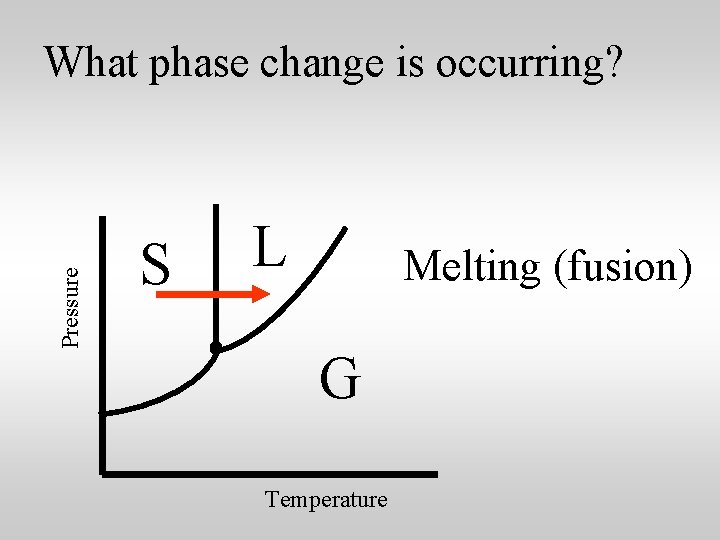
Pressure What phase change is occurring? S L Melting (fusion) G Temperature

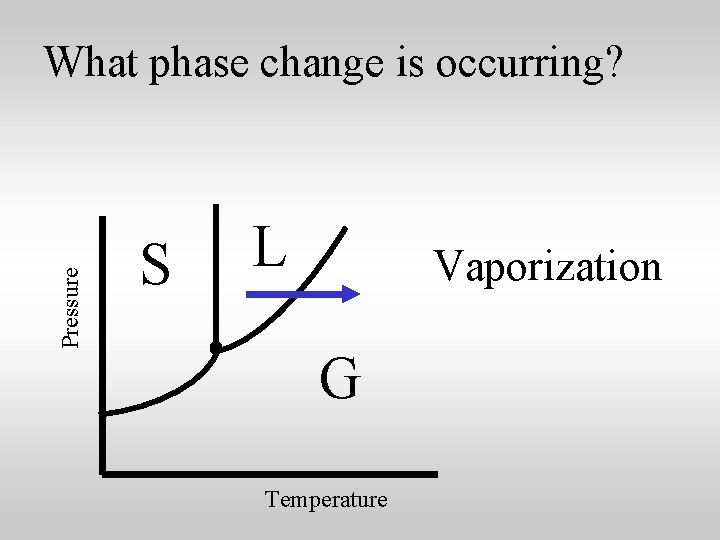
Pressure What phase change is occurring? S L Vaporization G Temperature

Pressure What phase change is occurring? S L Condensation G Temperature

Pressure What phase change is occurring? S L Sublimation G Temperature

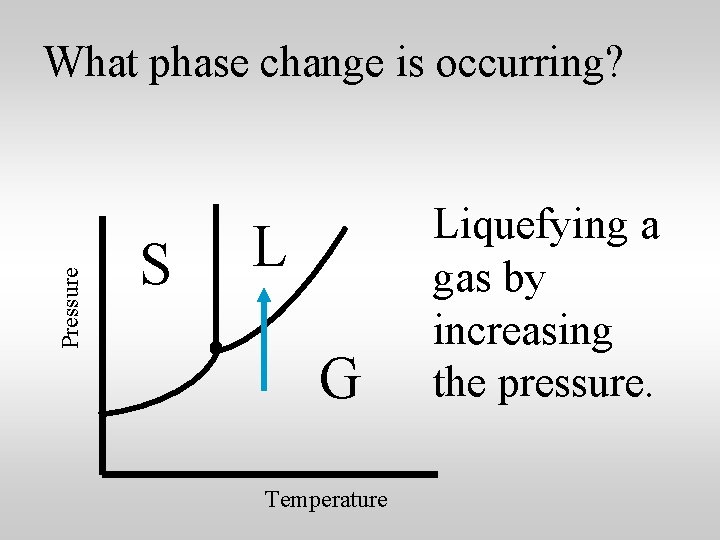
Pressure What phase change is occurring? S L G Temperature Liquefying a gas by increasing the pressure.

Part Five Heating and Cooling Curves

A process that absorbs heat is called endothermic. A process that gives off heat is called exothermic.

Melting (fusion) Endothermic: Vaporization Heat is absorbed. Sublimation Freezing Exothermic: Condensation Heat is released. Deposition

Investigate: Either recall an earlier experiment, or design an experiment to look at the temperature of water as phase changes take place.

Investigate: The following are suggested procedures you could use to record the temperature of water at regular intervals. Note: Hot plates and boiling water can cause severe burns.

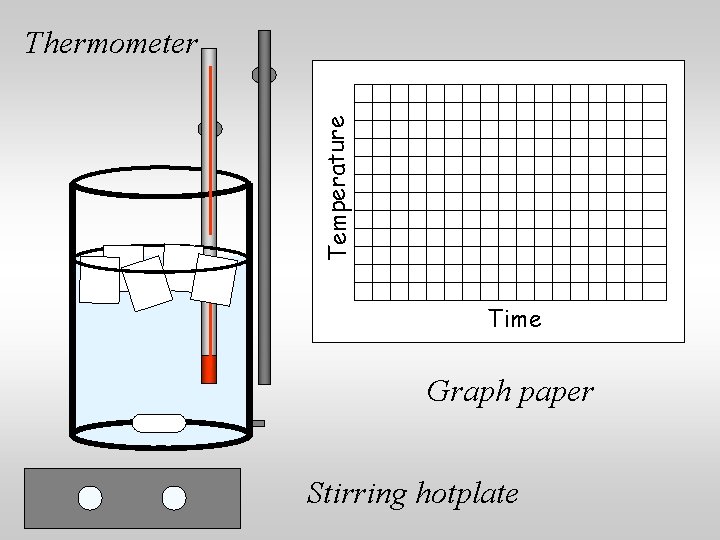
Investigate: 1. Clamp a thermometer with the bulb in a mixture of ice and water in a beaker on a hot plate. (The hot plate is off. ) 2. Allow the temperature to equilibrate. 3. Turn on the hot plate and continue to record temperature at regular intervals until some of the water boils away. 4. Plot temperature as a function of time.

Temperature Thermometer Time Graph paper Stirring hotplate

Thermometer Temperature You could use a thermometer and plot the temperature on graph paper or on Time a computer. Graph paper Stirring hotplate

Temperature Thermometer Time Stirring hotplate

Or. Cyou 0. 0 could use a thermometer probe connected to an Time interface and a computer CBL, Lab. Pro, to plotorthe temperature. Temperature probe computer Stirring hotplate

0. 0 C Temperature probe CBL, Lab. Pro, or computer Stirring hotplate Time

Phase changes occur at a single temperature. Water freezes and ice melts at 0 C. At sea level, water boils and steam condenses at 100 C.

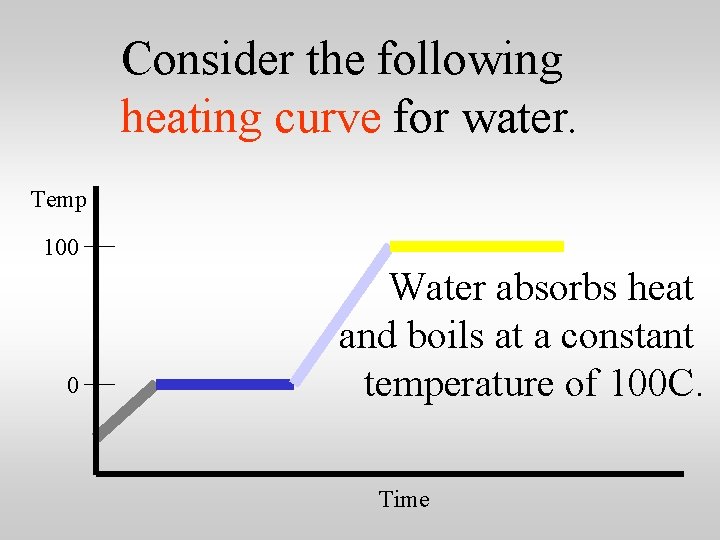
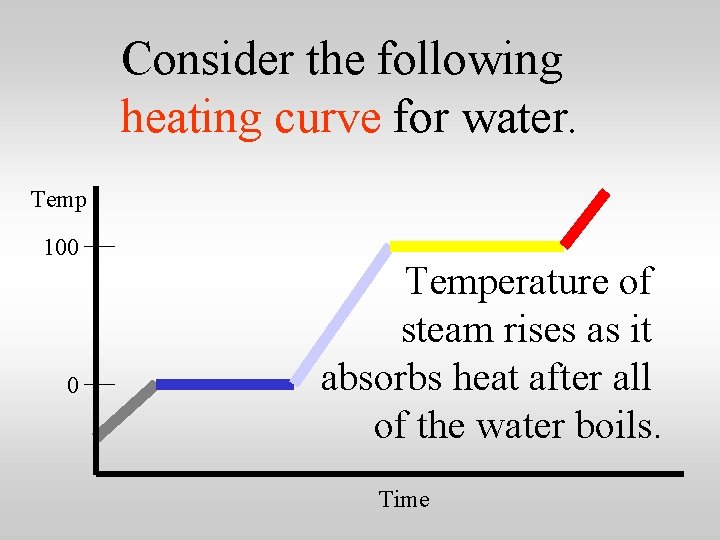
Consider the following heating curve for water. Temp 100 0 Time

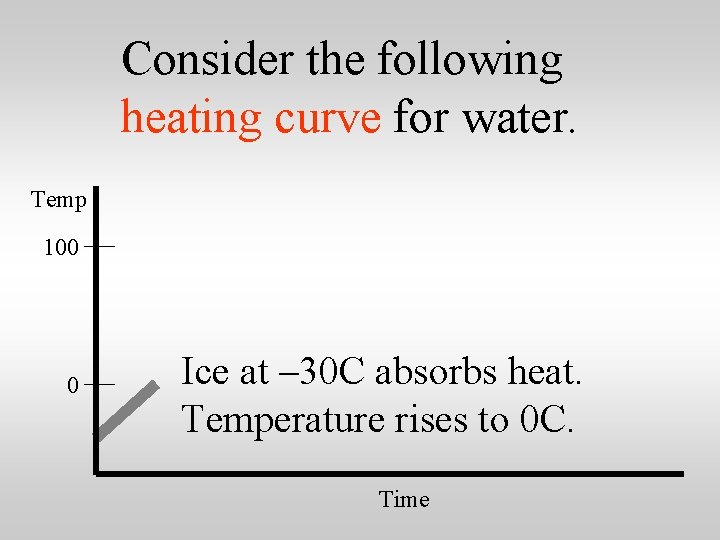
Consider the following heating curve for water. Temp 100 0 Ice at – 30 C absorbs heat. Temperature rises to 0 C. Time

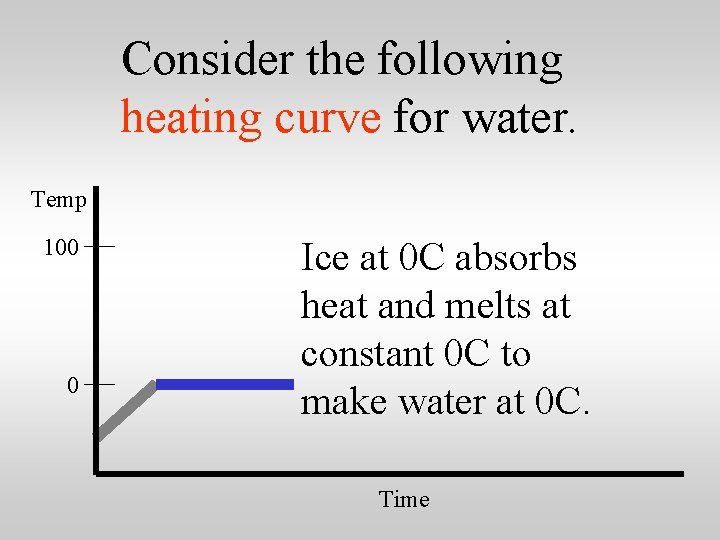
Consider the following heating curve for water. Temp 100 0 Ice at 0 C absorbs heat and melts at constant 0 C to make water at 0 C. Time

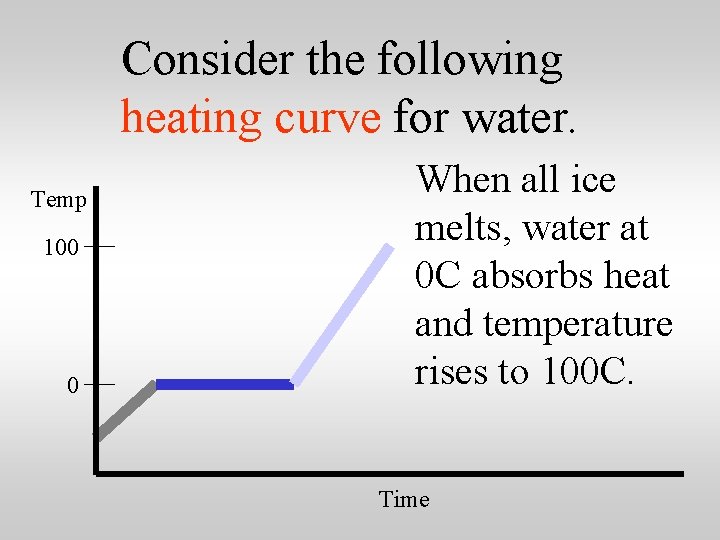
Consider the following heating curve for water. Temp 100 0 When all ice melts, water at 0 C absorbs heat and temperature rises to 100 C. Time

Consider the following heating curve for water. Temp 100 0 Water absorbs heat and boils at a constant temperature of 100 C. Time

Consider the following heating curve for water. Temp 100 0 Temperature of steam rises as it absorbs heat after all of the water boils. Time

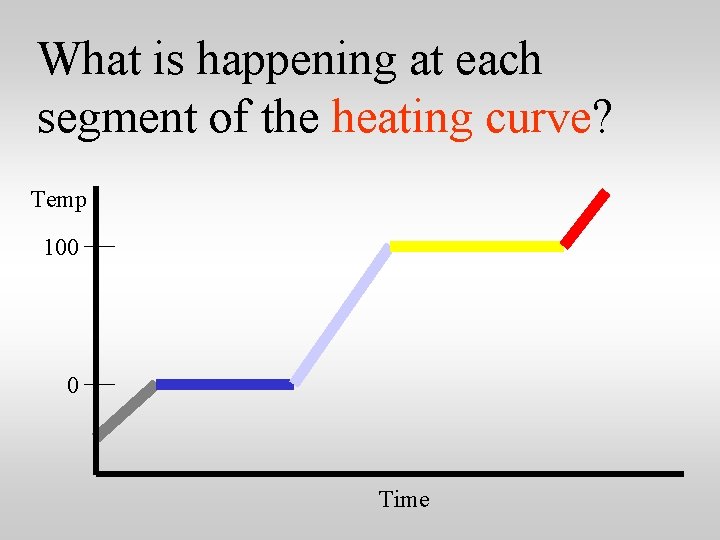
What is happening at each segment of the heating curve? Temp 100 0 Time

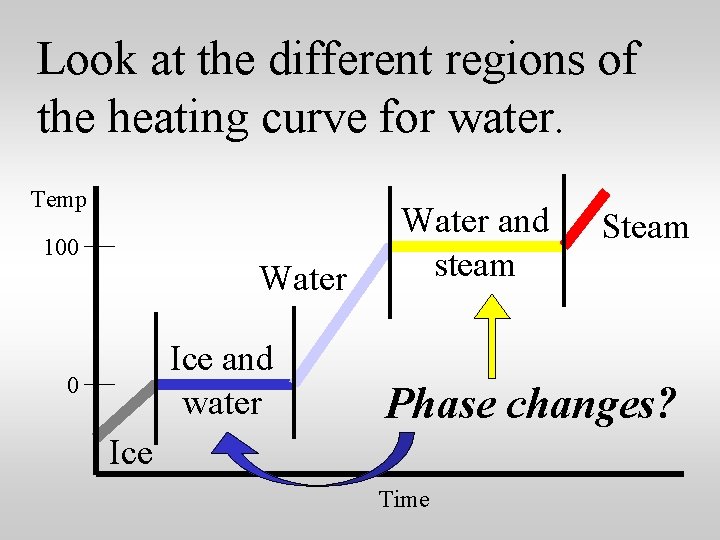
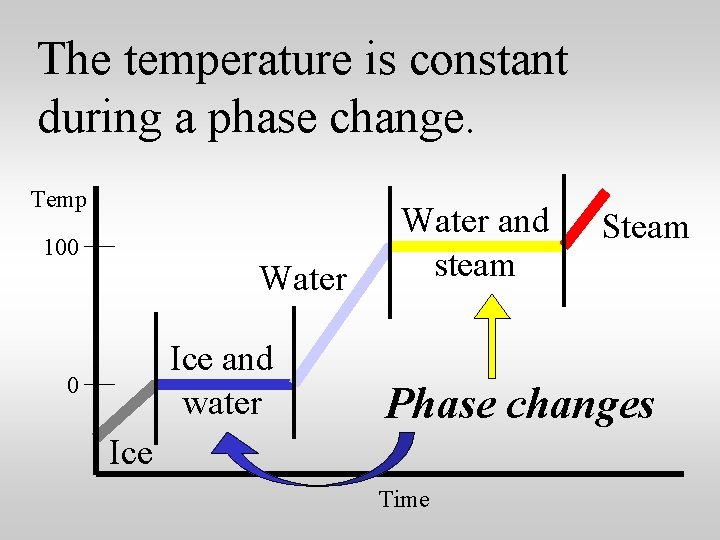
Look at the different regions of the heating curve for water. Temp 100 Water Ice and water 0 Water and steam Steam Phase changes? Ice Time

The temperature is constant during a phase change. Temp 100 Water Ice and water 0 Water and steam Steam Phase changes Ice Time

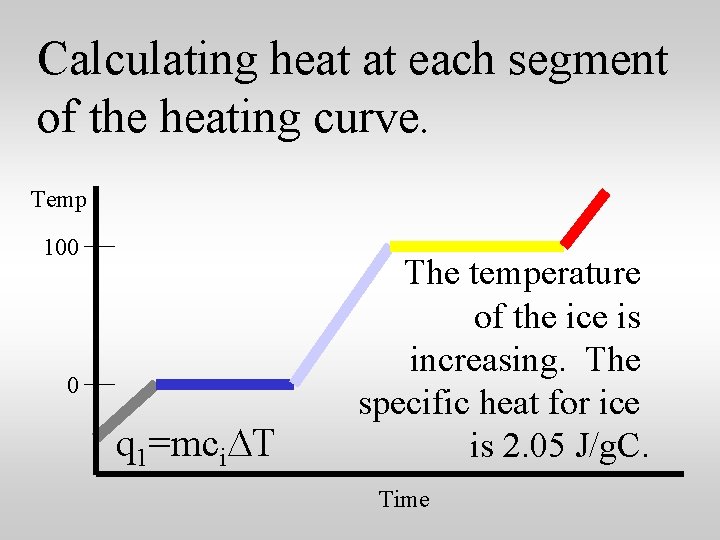
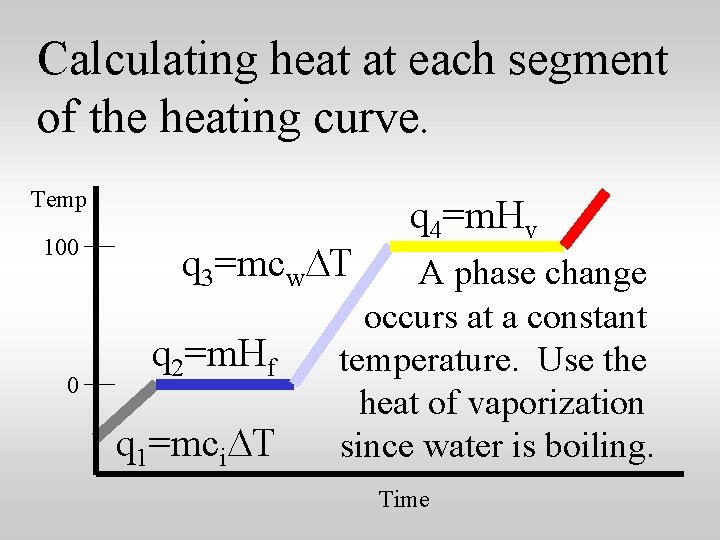
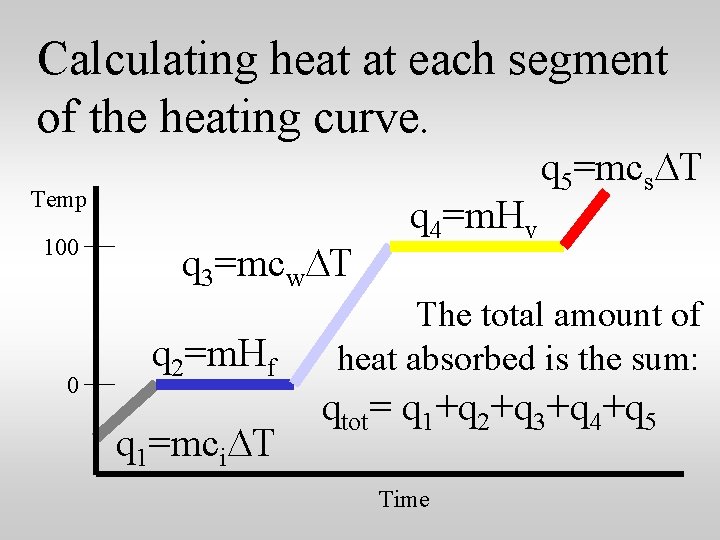
Calculating heat at each segment of the heating curve. Temp 100 0 q 1=mci. DT The temperature of the ice is increasing. The specific heat for ice is 2. 05 J/g. C. Time

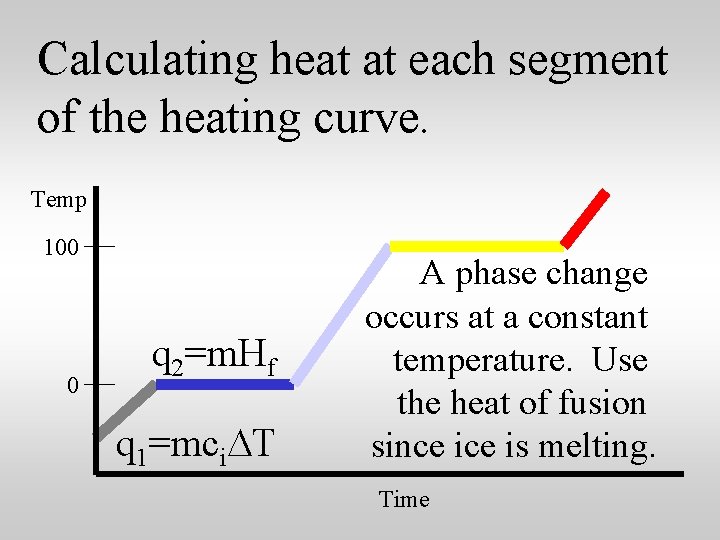
Calculating heat at each segment of the heating curve. Temp 100 0 q 2=m. Hf q 1=mci. DT A phase change occurs at a constant temperature. Use the heat of fusion since is melting. Time

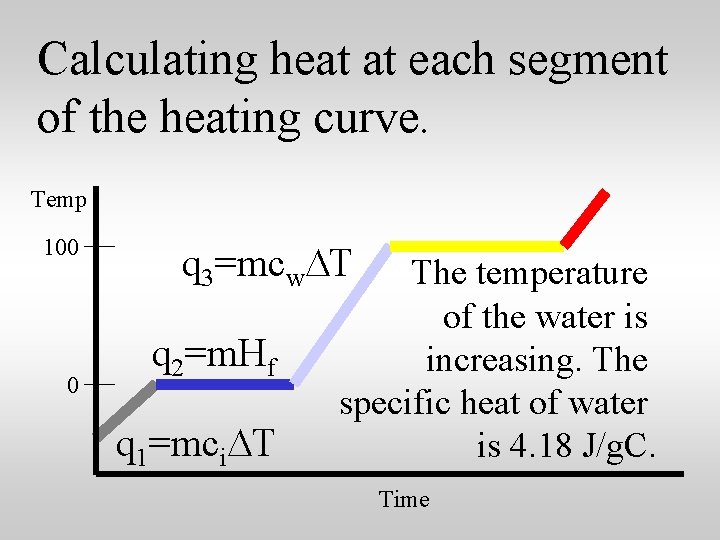
Calculating heat at each segment of the heating curve. Temp 100 0 q 3=mcw. DT q 2=m. Hf q 1=mci. DT The temperature of the water is increasing. The specific heat of water is 4. 18 J/g. C. Time

Calculating heat at each segment of the heating curve. Temp 100 0 q 3=mcw. DT q 2=m. Hf q 1=mci. DT q 4=m. Hv A phase change occurs at a constant temperature. Use the heat of vaporization since water is boiling. Time

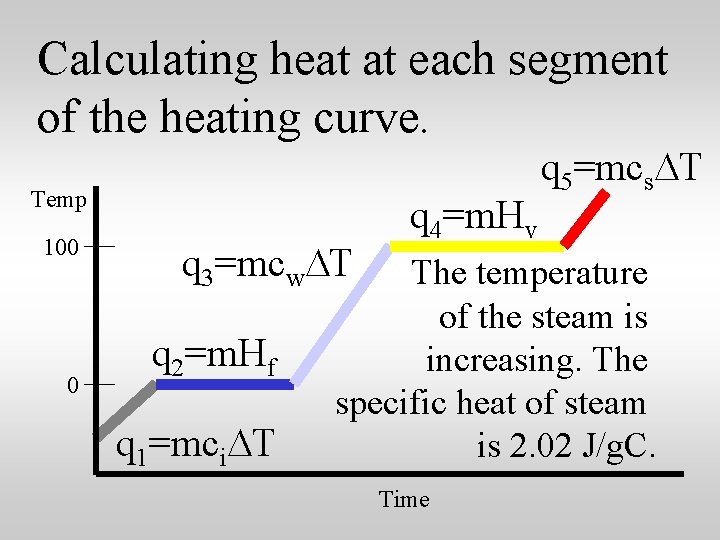
Calculating heat at each segment of the heating curve. Temp 100 0 q 3=mcw. DT q 2=m. Hf q 1=mci. DT q 4=m. Hv q 5=mcs. DT The temperature of the steam is increasing. The specific heat of steam is 2. 02 J/g. C. Time

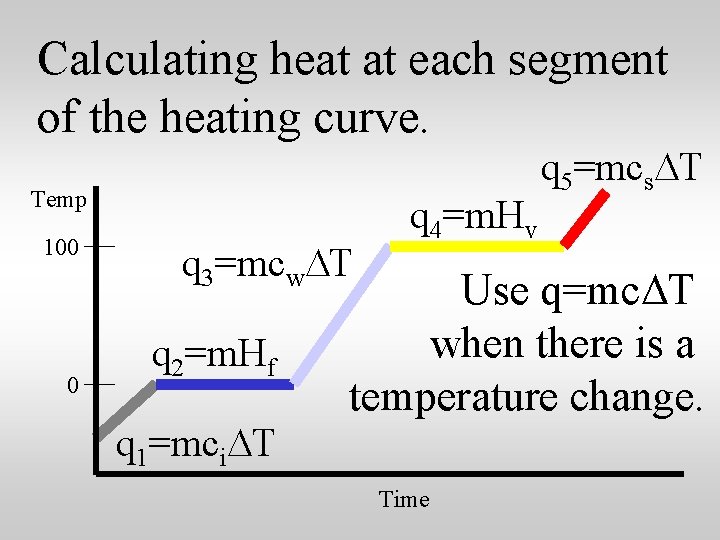
Calculating heat at each segment of the heating curve. Temp 100 0 q 3=mcw. DT q 2=m. Hf q 1=mci. DT q 4=m. Hv q 5=mcs. DT Use q=mc. DT when there is a temperature change. Time

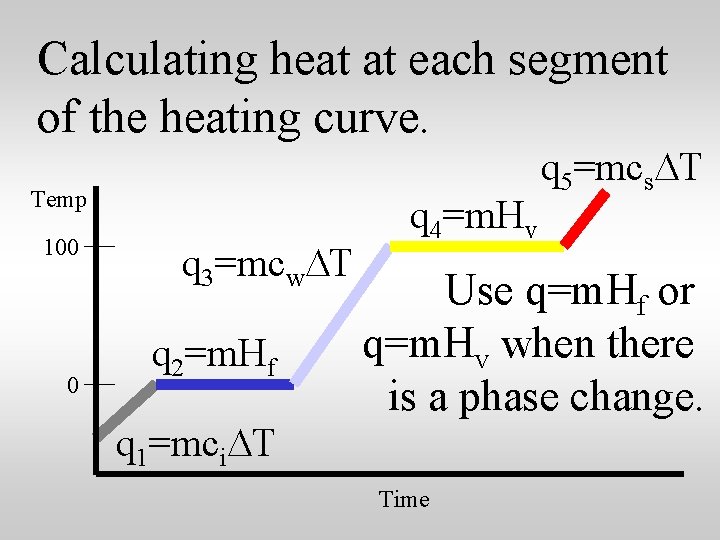
Calculating heat at each segment of the heating curve. Temp 100 0 q 3=mcw. DT q 2=m. Hf q 1=mci. DT q 4=m. Hv q 5=mcs. DT Use q=m. Hf or q=m. Hv when there is a phase change. Time

Calculating heat at each segment of the heating curve. Temp 100 0 q 3=mcw. DT q 2=m. Hf q 1=mci. DT q 4=m. Hv q 5=mcs. DT The total amount of heat absorbed is the sum: qtot= q 1+q 2+q 3+q 4+q 5 Time

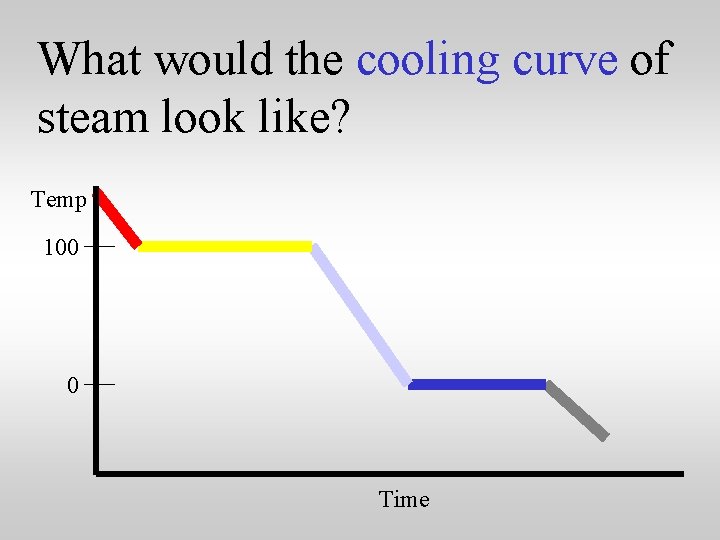
What would the cooling curve of steam look like? Temp 100 0 Time

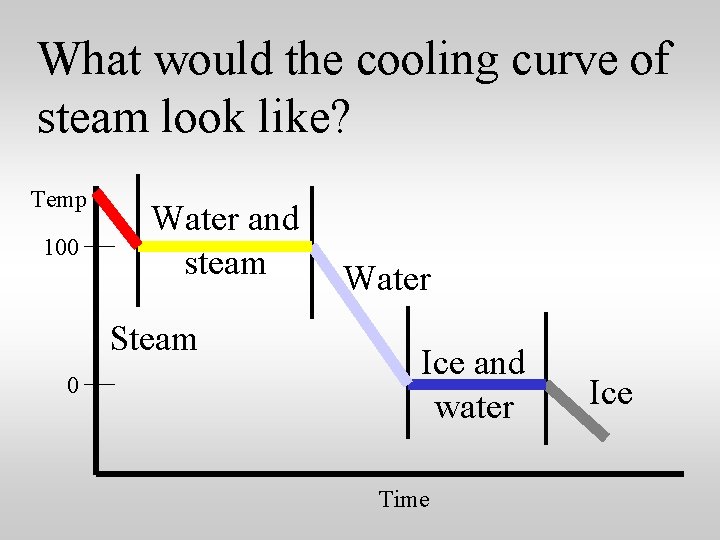
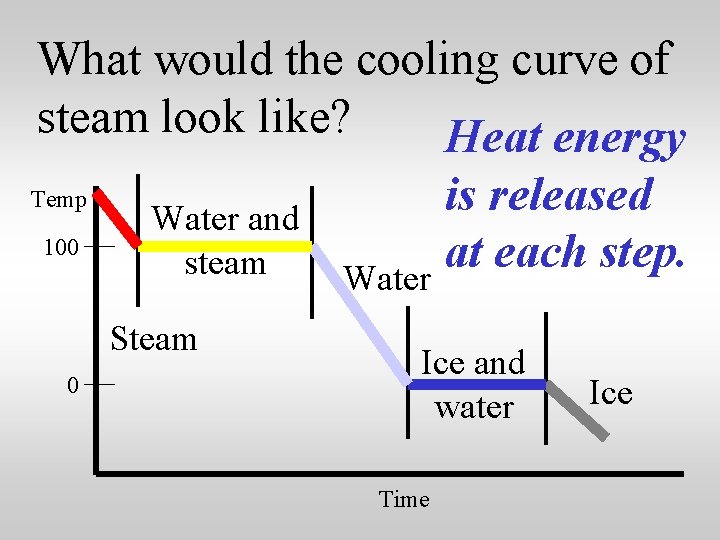
What would the cooling curve of steam look like? Temp 100 Water and steam Steam 0 Water Ice and water Time Ice

What would the cooling curve of steam look like? Heat energy Temp 100 Water and steam Steam 0 is released at each step. Water Ice and water Time Ice

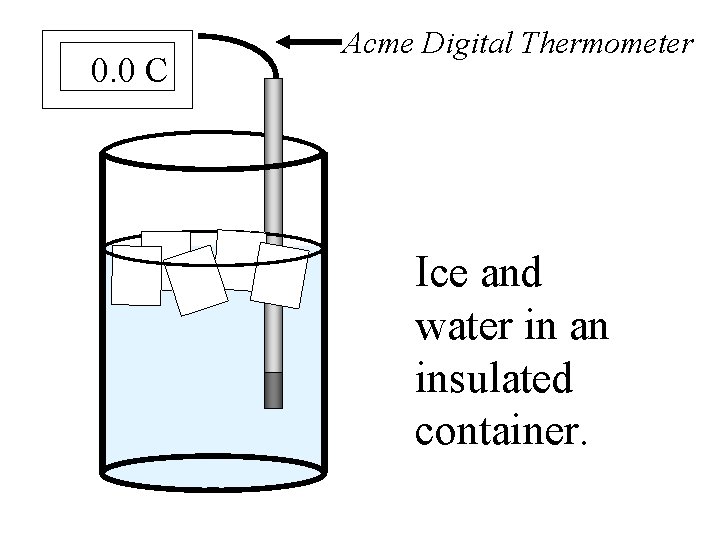
There is something else special about a mixture of ice and water. Suppose ice and water were placed into a perfectly insulated container. The mixture would stay at a constant zero degrees Celsius by establishing an equilibrium.

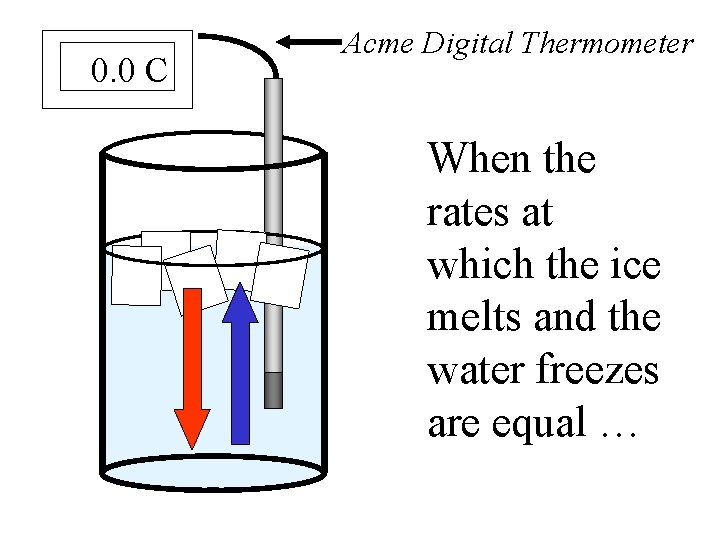
An ice/water equilibrium occurs when the rate at which water freezes is equal to the rate at which ice melts. The amount of ice and water will never change. If the container is completely insulated.

0. 0 C Acme Digital Thermometer Ice and water in an insulated container.

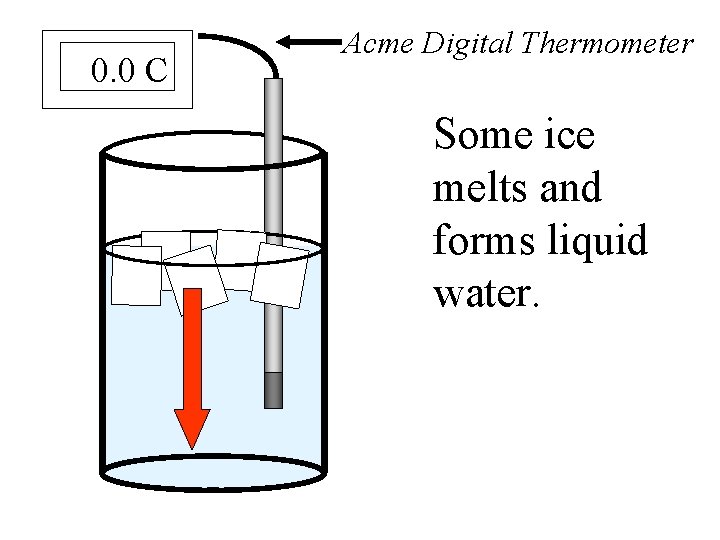
0. 0 C Acme Digital Thermometer Some ice melts and forms liquid water.

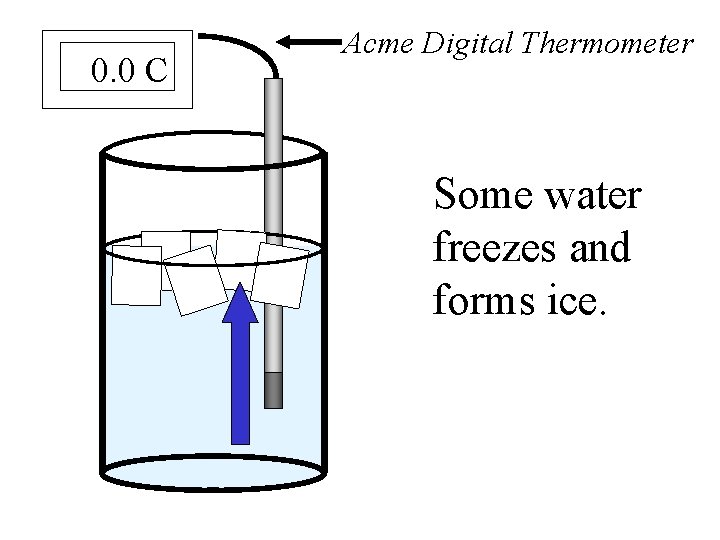
0. 0 C Acme Digital Thermometer Some water freezes and forms ice.

0. 0 C Acme Digital Thermometer When the rates at which the ice melts and the water freezes are equal …

0. 0 C Acme Digital Thermometer … an equilibrium is established.

0. 0 C Acme Digital Thermometer The amounts of ice and water will remain constant…

0. 0 C Acme Digital Thermometer …and the mixture of ice and water will remain at a constant 0 C.

0. 0 C Acme Digital Thermometer A mixture of ice and water can be used to calibrate a thermometer at 0 C.

Questions 1. Ice and water are placed in an insulated container. What will be the equilibrium temperature? 2. A substance freezes at -80. 0 C. At what temperature does it melt?

Questions 3. A liquid gradually turns solid at a constant temperature. Is heat being added, or removed? 4. How does melting snow affect the air temperature?

Questions 5. When water vapor condenses to form liquid water, is heat released or absorbed? 6. What is the connection between condensing water vapor and updrafts in thunderstorms?

Questions 7. Explain how sweating cools your body. 8. Explain how liquid water evaporating from a roadway can cause ice to form on the road.

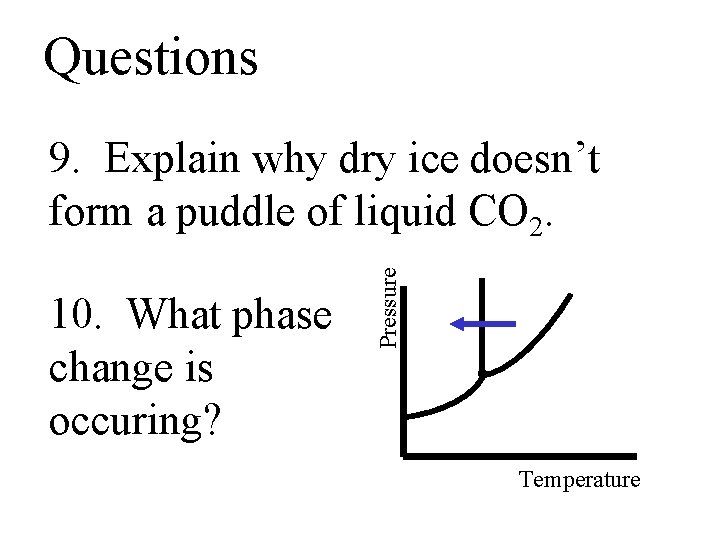
Questions 10. What phase change is occuring? Pressure 9. Explain why dry ice doesn’t form a puddle of liquid CO 2. Temperature
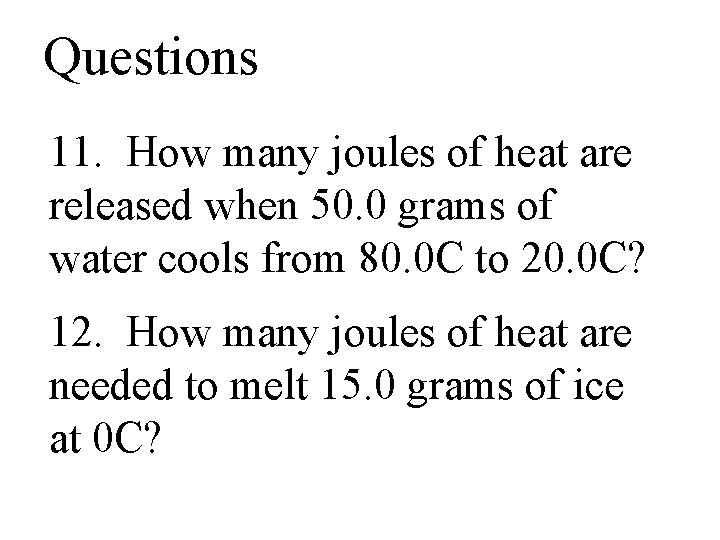
Questions 11. How many joules of heat are released when 50. 0 grams of water cools from 80. 0 C to 20. 0 C? 12. How many joules of heat are needed to melt 15. 0 grams of ice at 0 C?
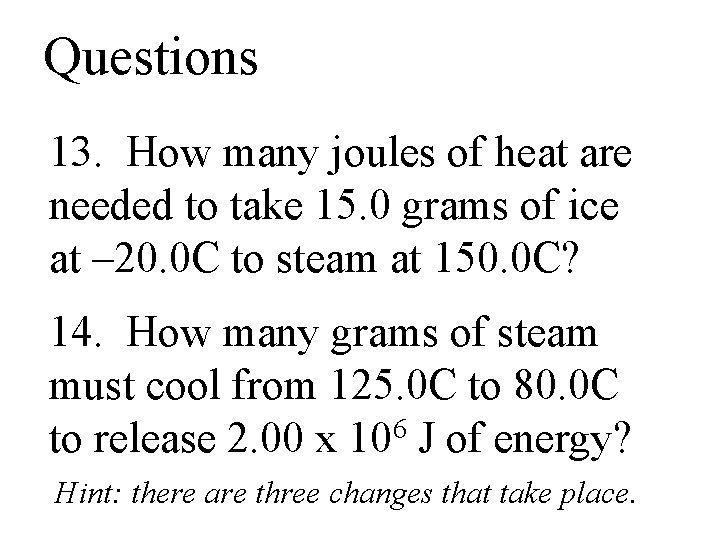
Questions 13. How many joules of heat are needed to take 15. 0 grams of ice at – 20. 0 C to steam at 150. 0 C? 14. How many grams of steam must cool from 125. 0 C to 80. 0 C to release 2. 00 x 106 J of energy? Hint: there are three changes that take place.

Heat Deposition Melting Temperature Phase change Equilibrium Joule Phase diagram Vaporization Heating curve Calorie Condensation Sublimation Freezing Celsius Boiling