Heat Section 1 Preview Section 1 Temperature and




















- Slides: 37

Heat Section 1 Preview Section 1 Temperature and Thermal Equilibrium Section 2 Defining Heat Section 3 Changes in Temperature and Phase © Houghton Mifflin Harcourt Publishing Company

Heat TEKS Section 1 The student is expected to: 6 E describe how the macroscopic properties of a thermodynamic system such as temperature, specific heat, and pressure are related to the molecular level of matter, including kinetic or potential energy of atoms © Houghton Mifflin Harcourt Publishing Company

Heat Section 1 What do you think? • Suppose you have two cups of water. One is hot and the other is cold. – How is the cold water different from the hot water? • Describe the motion of the molecules in each. – What changes would occur if the hot water was changed into steam? • What are the common scales used to measure temperature? – When is each scale generally used? – All scales use degrees to measure temperature. Which scale has the largest degrees? Explain. © Houghton Mifflin Harcourt Publishing Company

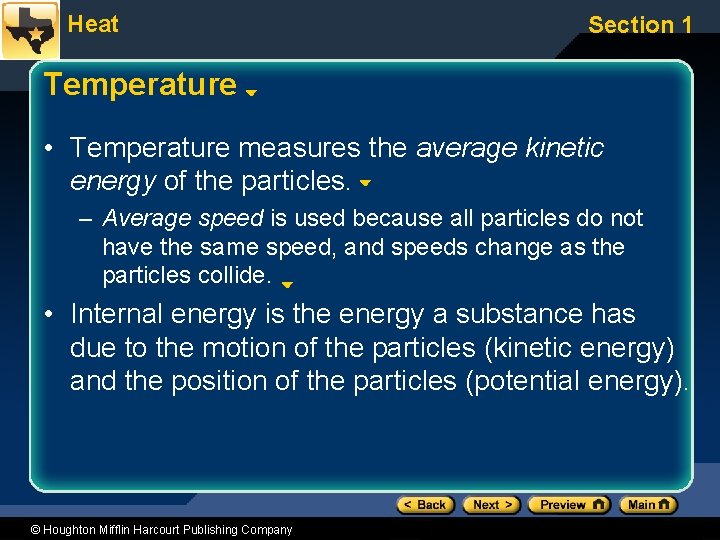
Heat Section 1 Temperature • Temperature measures the average kinetic energy of the particles. – Average speed is used because all particles do not have the same speed, and speeds change as the particles collide. • Internal energy is the energy a substance has due to the motion of the particles (kinetic energy) and the position of the particles (potential energy). © Houghton Mifflin Harcourt Publishing Company

Heat Section 1 Forms of Internal Energy Click below to watch the Visual Concept © Houghton Mifflin Harcourt Publishing Company

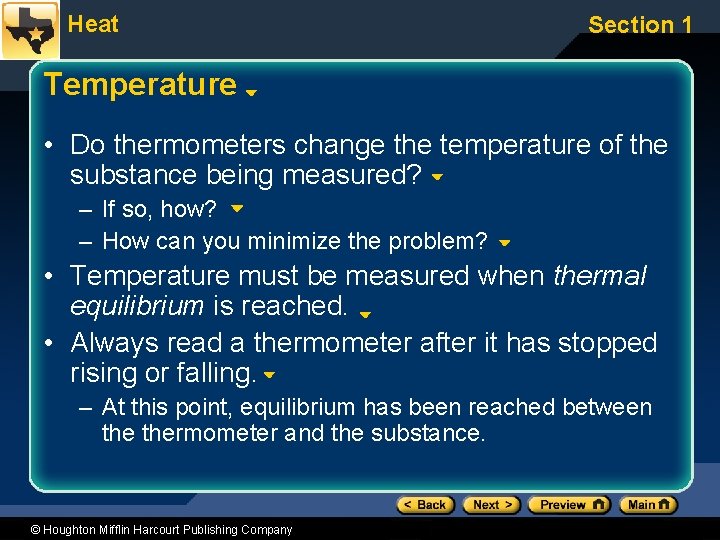
Heat Section 1 Temperature • Do thermometers change the temperature of the substance being measured? – If so, how? – How can you minimize the problem? • Temperature must be measured when thermal equilibrium is reached. • Always read a thermometer after it has stopped rising or falling. – At this point, equilibrium has been reached between thermometer and the substance. © Houghton Mifflin Harcourt Publishing Company

Heat Section 1 Thermal Expansion Click below to watch the Visual Concept © Houghton Mifflin Harcourt Publishing Company

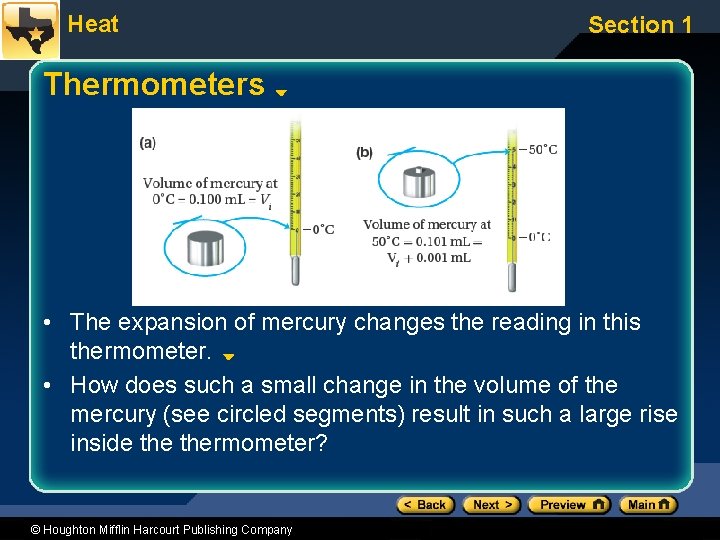
Heat Section 1 Thermometers • The expansion of mercury changes the reading in this thermometer. • How does such a small change in the volume of the mercury (see circled segments) result in such a large rise inside thermometer? © Houghton Mifflin Harcourt Publishing Company

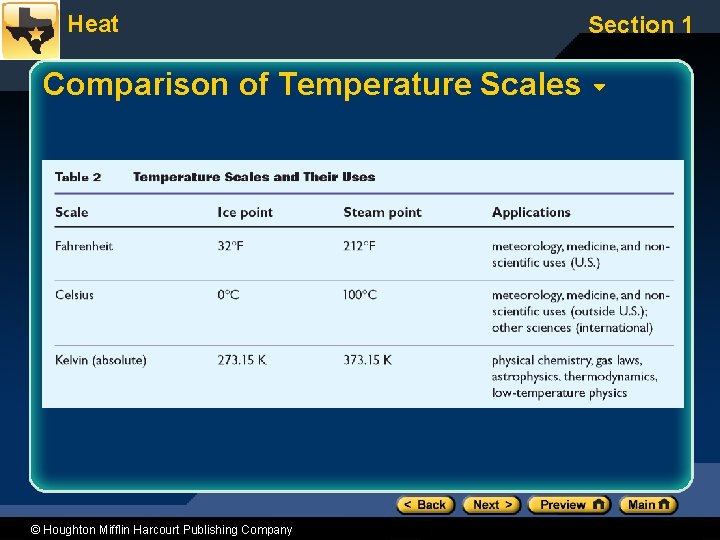
Heat Section 1 Thermometers • Calibration depends on fixed temperatures. • Three common temperature scales used: – Fahrenheit for weather and medicine (U. S. ) – Celsius for work in science – Kelvin or absolute for many scientific laws © Houghton Mifflin Harcourt Publishing Company

Heat Comparison of Temperature Scales © Houghton Mifflin Harcourt Publishing Company Section 1

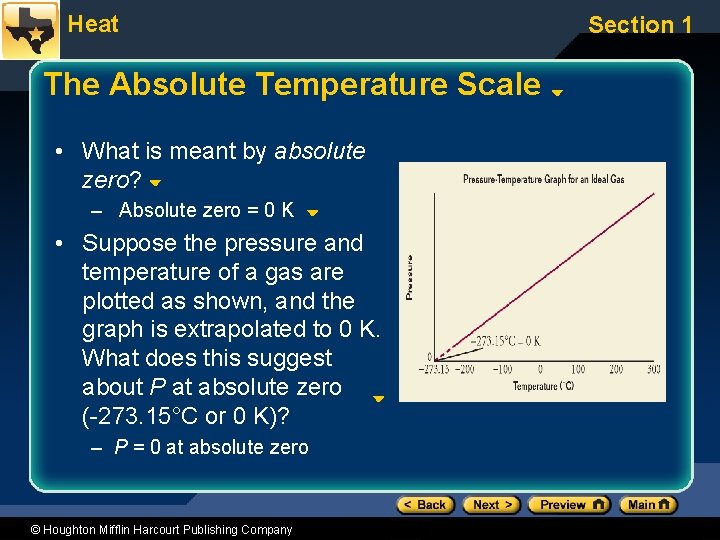
Heat The Absolute Temperature Scale • What is meant by absolute zero? – Absolute zero = 0 K • Suppose the pressure and temperature of a gas are plotted as shown, and the graph is extrapolated to 0 K. What does this suggest about P at absolute zero (-273. 15°C or 0 K)? – P = 0 at absolute zero © Houghton Mifflin Harcourt Publishing Company Section 1

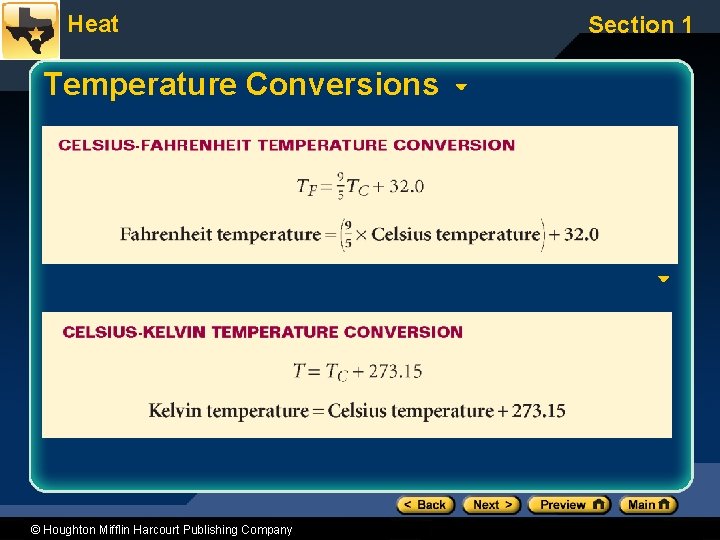
Heat Temperature Conversions © Houghton Mifflin Harcourt Publishing Company Section 1

Heat Section 1 Classroom Practice Problems • One day it was -40°C at the top of Mont Blanc and -40°F at the top of Mount Whitney. Which place was colder? – Answer: Neither (-40°C = -40°F) • What is the Fahrenheit temperature equivalent to absolute zero? – Answer: -459. 67°F • What is the Celsius temperature on a hot summer day when the temperature is 100. °F? – Answer: 37. 8°C © Houghton Mifflin Harcourt Publishing Company

Heat Section 1 Now what do you think? • Suppose you have two cups of water. One is hot and the other is cold. – How is the cold water different from the hot water? • Describe the motion of the molecules in each. – What changes would occur if the hot water was changed into steam? • What are the common scales used to measure temperature? – When is each scale generally used? – All scales use degrees to measure temperature. Which scale has the largest degrees? Explain. © Houghton Mifflin Harcourt Publishing Company

Heat TEKS Section 2 The student is expected to: 6 E describe how the macroscopic properties of a thermodynamic system such as temperature, specific heat, and pressure are related to the molecular level of matter, including kinetic or potential energy of atoms 6 F contrast and give examples of different processes of thermal energy transfer, including conduction, convection, and radiation © Houghton Mifflin Harcourt Publishing Company

Heat Section 2 What do you think? • Internal energy is the energy due to the kinetic and potential energy of the particles. – Does the ice water or an equal quantity of hot chocolate have greater internal energy? Why? – Which has more internal energy, a gallon of cold water or a drop of hot chocolate? – How will the internal energy of the water and hot chocolate change over time? • How will this change occur? © Houghton Mifflin Harcourt Publishing Company

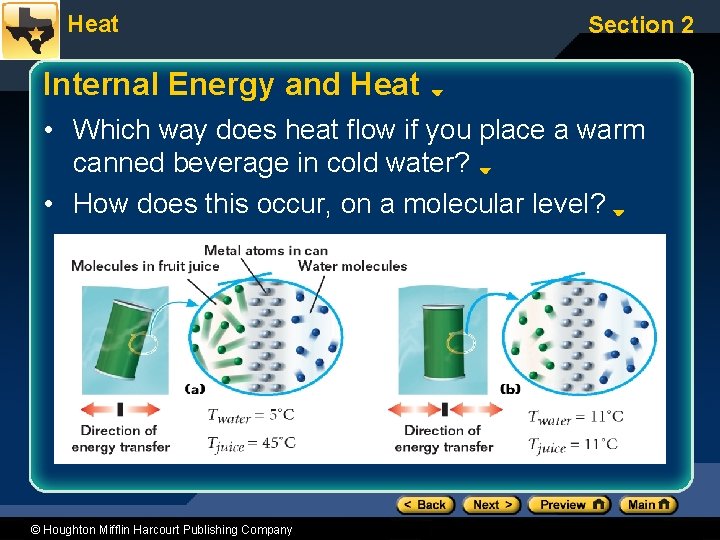
Heat Section 2 Internal Energy and Heat • Internal Energy (U) is the energy contained within the particles of a substance. • Heat (Q) is the internal energy transferred between objects. – Heat always moves from a higher-temperature object to a lower-temperature object. – The rate of transfer depends on the difference in temperature. • The greater the temperature difference, the greater the rate of energy transfer (if other factors are equal). © Houghton Mifflin Harcourt Publishing Company

Heat Section 2 Internal Energy and Heat • Which way does heat flow if you place a warm canned beverage in cold water? • How does this occur, on a molecular level? © Houghton Mifflin Harcourt Publishing Company

Heat Section 2 Temperature and Heat Click below to watch the Visual Concept © Houghton Mifflin Harcourt Publishing Company

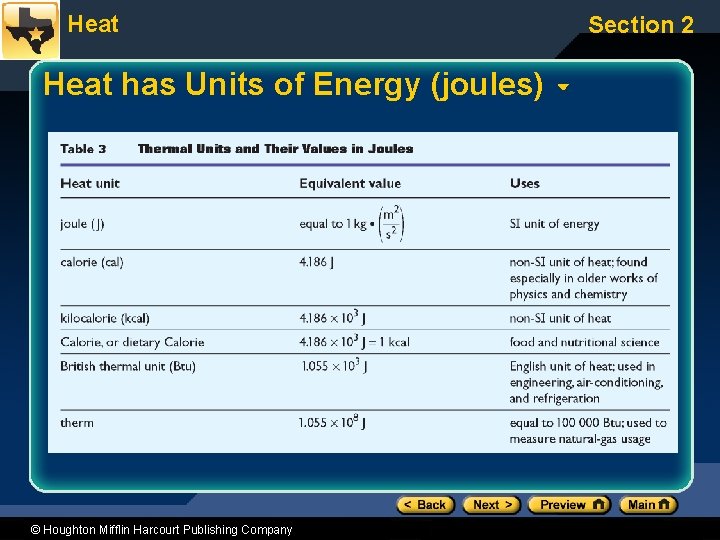
Heat has Units of Energy (joules) © Houghton Mifflin Harcourt Publishing Company Section 2

Heat Section 2 Heat Transfer • In what three ways can internal energy be transferred from a hot object to a colder object? – Conduction is the transfer of heat through a substance by molecule to molecule contact. • Metals are good conductors. • Styrofoam is a good insulator. – Convection is the transfer of energy by the movement of a fluid. • Hot air in a room rises and cold air moves in to replace it. – Radiation is the transfer of energy by electromagnetic waves. • No matter is transferred, only energy. © Houghton Mifflin Harcourt Publishing Company

Heat Section 2 Comparing Convection, Conduction, and Radiation Click below to watch the Visual Concept © Houghton Mifflin Harcourt Publishing Company

Heat Section 2 Heat and Work • Work can be changed into internal energy (U). – Rub your hands together and you’ll feel the increase in internal energy produced by your work. – Pull a nail from a piece of wood and the nail is hot. • Mechanical energy (PE + KE) is conserved when there is no friction. • Total energy, including internal energy, is always conserved. © Houghton Mifflin Harcourt Publishing Company

Heat Section 2 Energy Conservation • Any loss of one type is balanced by a gain in the other types of energy. • Predict the sign (+, -, or 0) for the change in each quantity when: – A child slides down a plastic playground slide – A car applies the brakes to stop on a level road © Houghton Mifflin Harcourt Publishing Company

Heat Section 2 Now what do you think? – Does the ice water or an equal quantity of hot chocolate have greater internal energy? Why? – Which has more internal energy, a gallon of cold water or a drop of hot chocolate? – How will the internal energy of the water and hot chocolate change over time? • How will this change occur? © Houghton Mifflin Harcourt Publishing Company

Heat TEKS Section 3 The student is expected to: 6 E describe how the macroscopic properties of a thermodynamic system such as temperature, specific heat, and pressure are related to the molecular level of matter, including kinetic or potential energy of atoms © Houghton Mifflin Harcourt Publishing Company

Heat Section 3 What do you think? • What property of water makes it so useful as a coolant in automobiles, nuclear reactors, and other machinery? • How does it differ from other liquids regarding its ability to cool substances? • Why do you feel cool when getting out of a warm swimming pool on a hot day? • How do you feel if it is windy out? Why? • How do you feel if it is an indoor pool? Why? © Houghton Mifflin Harcourt Publishing Company

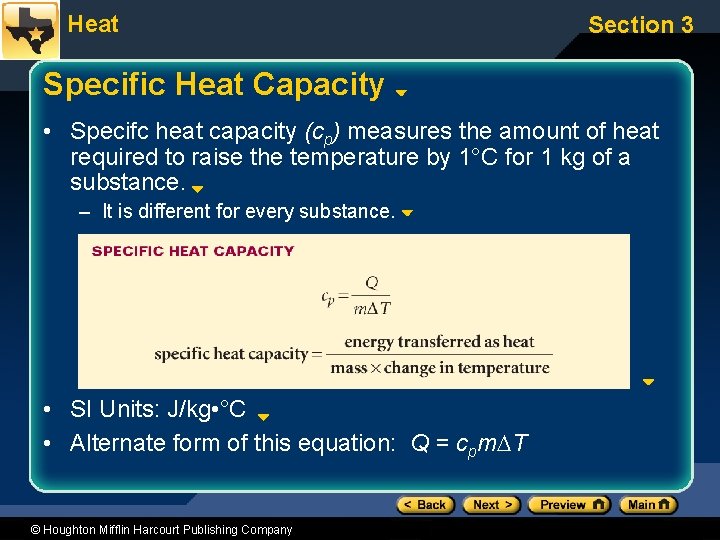
Heat Section 3 Specific Heat Capacity • Specifc heat capacity (cp) measures the amount of heat required to raise the temperature by 1°C for 1 kg of a substance. – It is different for every substance. • SI Units: J/kg • °C • Alternate form of this equation: Q = cpm T © Houghton Mifflin Harcourt Publishing Company

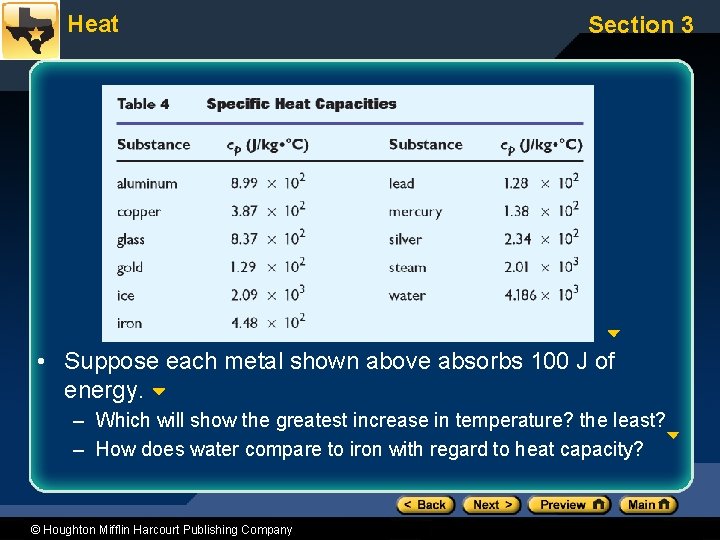
Heat Section 3 • Suppose each metal shown above absorbs 100 J of energy. – Which will show the greatest increase in temperature? the least? – How does water compare to iron with regard to heat capacity? © Houghton Mifflin Harcourt Publishing Company

Heat Calorimetry • Calorimetry is a problemsolving approach to heat transfer problems. – Conservation of energy – Qgained = -Qlost • A calorimeter is an insulated cup with water used for the experiment. – Ignore heat gained or lost by the cup, as it is small. © Houghton Mifflin Harcourt Publishing Company Section 3

Heat Section 3 Classroom Practice Problem • In a student’s experiment, a 0. 125 kg metal ball is placed in a calorimeter filled with 0. 150 kg of water at 21. 0°C. The initial temperature of the ball is 98. 5°C. After reaching equilibrium, the temperature is 27. 3°C. Find the specific heat capacity of the metal and use the table to determine the type of metal. – Answer: 444 J/kg • °C , very close to iron © Houghton Mifflin Harcourt Publishing Company

Heat Section 3 Classroom Practice Problem • A bathtub has 20. 0 kg of water at 60. 0°C and the bather wants the temperature to be 30. 0°C. How much 20. 0°C water must be added to the bath water to achieve the desired temperature? – Answer : 60. 0 kg © Houghton Mifflin Harcourt Publishing Company

Heat Section 3 Latent Heat • Latent heat is heat gained or lost during phase changes. – When substances melt, freeze, boil, condense, or sublime, the temperature does not change during the phase change. – Heat absorbed changes the potential energy of the particles. © Houghton Mifflin Harcourt Publishing Company

Heat Section 3 Latent Heat Click below to watch the Visual Concept © Houghton Mifflin Harcourt Publishing Company

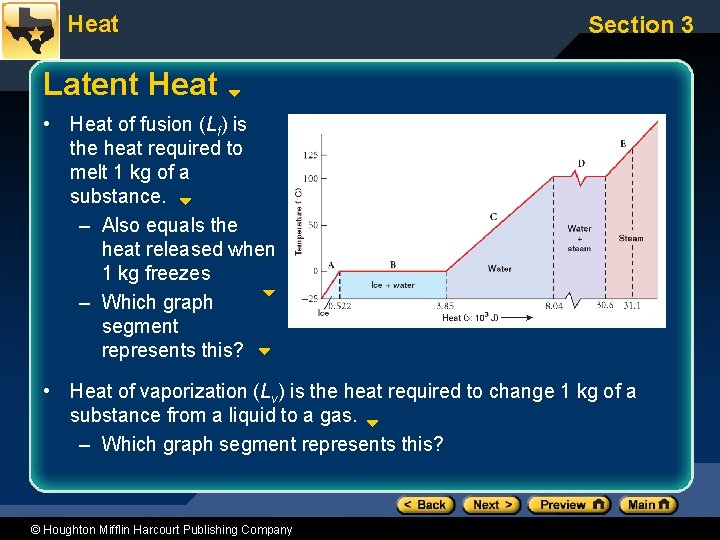
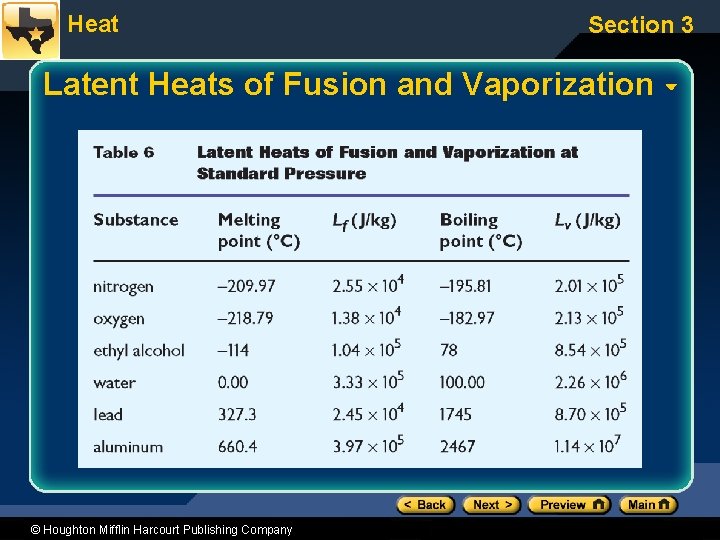
Heat Section 3 Latent Heat • Heat of fusion (Lf) is the heat required to melt 1 kg of a substance. – Also equals the heat released when 1 kg freezes – Which graph segment represents this? • Heat of vaporization (Lv) is the heat required to change 1 kg of a substance from a liquid to a gas. – Which graph segment represents this? © Houghton Mifflin Harcourt Publishing Company

Heat Section 3 Latent Heats of Fusion and Vaporization © Houghton Mifflin Harcourt Publishing Company

Heat Section 3 Now what do you think? • What property of water makes it so useful as a coolant in automobiles, nuclear reactors and other machinery? • How does it differ from other liquids regarding its ability to cool substances? • Why do you feel cool when getting out of a warm swimming pool on a hot day? • How do you feel if it is windy out? Why? • How do you feel if it is an indoor pool? Why? © Houghton Mifflin Harcourt Publishing Company