Heat Phase Change Latent Heat Latent Heat of



















- Slides: 26


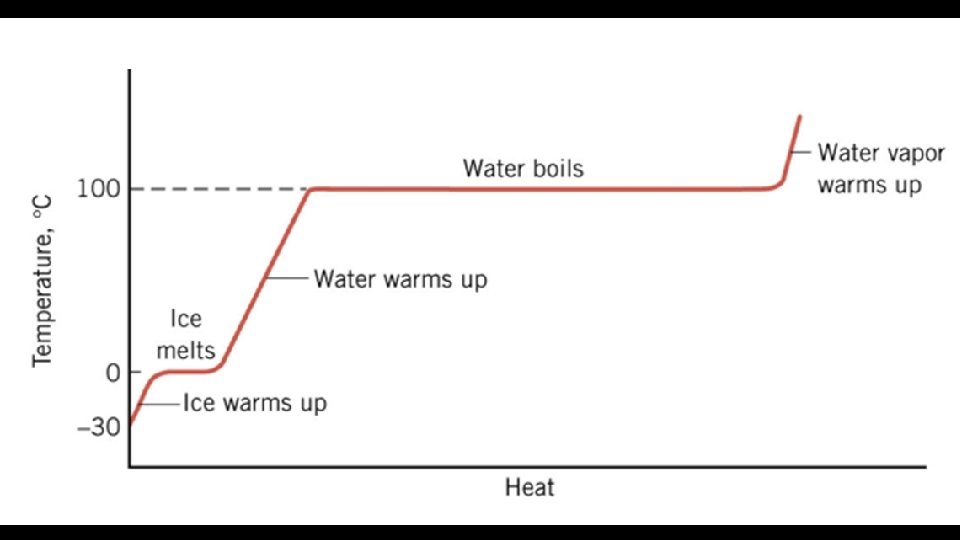
Heat & Phase Change (Latent Heat) • Latent Heat of Fusion • Energy required to melt a substance • (add heat = melting, remove = freezing)

Heat & Phase Change (Latent Heat) • Latent Heat of Vaporization • Energy required to vaporize a substance • (add = vaporizing, remove = condensing)

Heat & Phase Change (Latent Heat) • Heat Energy goes to overcoming the intermolecular forces instead of changing temperature. • Latent Heat of Fusion • When enough Heat Energy is removed the intermolecular forces are allowed to lock into a crystal structure. • Latent Heat of Vaporization • Heat Energy is added to break the intermolecular forces and separate the molecules even further.

Heat & Phase Change • Why does a liquid cool when some of it evaporates?
• Why does a liquid cool when some of it evaporates?

Heat & Phase Change • What are two things that effect evaporation rate?

Latent Heat • Latent Heat of Fusion (freezing / melting) Lfus (water) = 334 KJ/Kg • Latent Heat of Vaporization (boiling / condensing) • Lvap (water) = 2260 KJ/Kg • Example Problem – What Heat is required to raise 10 kg of Pure Water from -10 C to 110 C?

Practice • How much energy is needed to convert 50 grams of ice at 0° C to steam at 100° C? • How much energy is needed to convert 10 kg of ice at -20° C to steam at 150° C? • How much energy must be removed to change 500 grams of steam at 110° C to ice at -170° C?

Freezing Point Depression • What keeps the oceans from freezing? • What can keep the roads from freezing? • What keeps your car from overheating or freezing?

Boiling • What happens when something boils? • How does a solute raise the boiling point?

Freezing • What happens when something freezes? • How does a solute lower the freezing point?

Freezing Point Depression / Boiling Point Elevation • DRAW

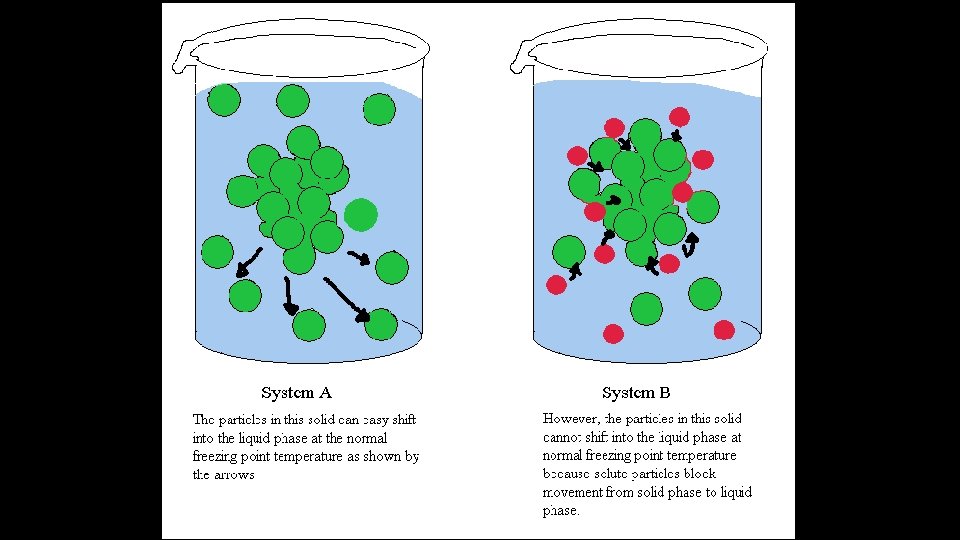
Boiling Point Elevation • A substance is dissolved in a solvent, like salt in water. • B. P. increases. • There are less liquid molecules at the surface that can turn into a gas. • The dissolved solid ions get in the way of the solvent molecules at the surface. • It takes a higher temperature (more energy) to get the liquid molecules to the surface and boil the solution.

Freezing Point Depression • A substance is dissolved in a solvent, like water. • F. P. decreases. • The solvent molecules begin to clump together (freeze) as energy is removed. • The dissolved solid ions get in the way of the solvent molecule intermolecular forces. • It takes more energy to be removed (lower temperature).


Freezing Point Depression / Boiling Point Elevation • ΔTf = (Kf) (m) • ΔTb = (Kb) (m) • ΔTf = Tf (pure solvent) – Tf (solution) • m = molality = moles of solute / kg of solvent • Kf = Freezing point depression constant (solvent)

Freezing Point Depression / Boiling Point Elevation • ΔTf = (Kf) (m) • ΔTb = (Kb) (m) • ΔT = C • m = mol / Kg • K = Kg C / mol

WATER • Kf = 1. 86 Kg C / mol • Kb = 0. 512 Kg C / mol

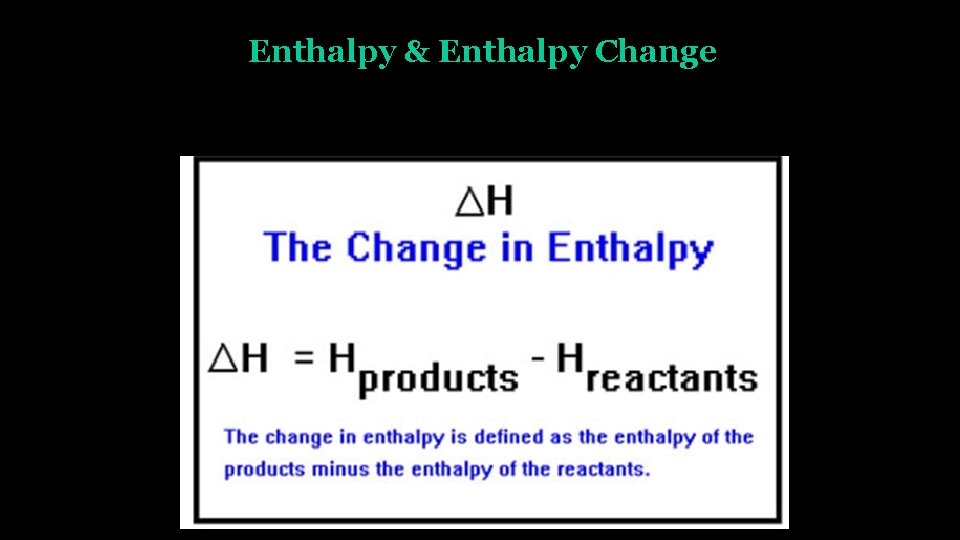
Enthalpy & Enthalpy Change (ΔH) • H = Q + PV • ΔH = Q + ΔPV • ΔH = Q (for constant pressure system) • Most reactions take place at constant pressure (atmospheric pressure).

Enthalpy & Enthalpy Change

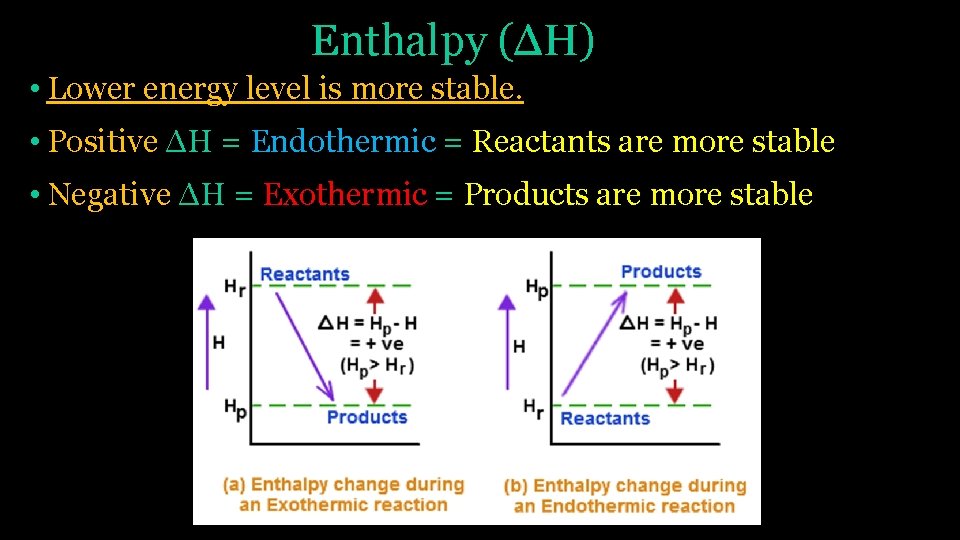
Enthalpy (ΔH) • Lower energy level is more stable. • Positive ΔH = Endothermic = Reactants are more stable • Negative ΔH = Exothermic = Products are more stable

Chemical Potential • Chemical Potential Energy • Energy stored in the bonds of molecules • Lower chemical potential is more stable • Relate to Gravitational Potential Energy • Cliff Diver

• Max Chemical Potential = top of the cliff • Unreacted Chemicals • Min Chemical Potential = bottom of the cliff • Reaction has occurred • Exothermic

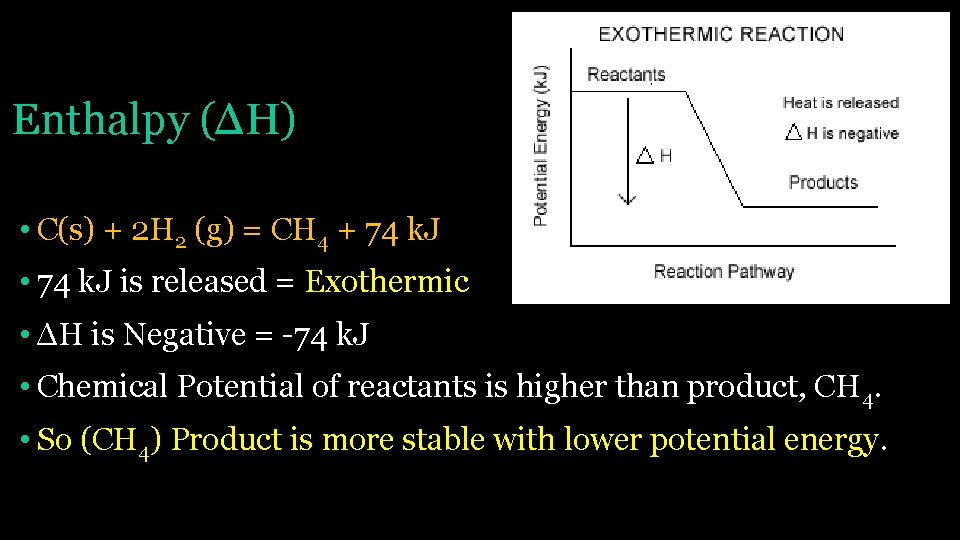
Enthalpy (ΔH) • C(s) + 2 H 2 (g) = CH 4 + 74 k. J • 74 k. J is released = Exothermic • ΔH is Negative = -74 k. J • Chemical Potential of reactants is higher than product, CH 4. • So (CH 4) Product is more stable with lower potential energy.

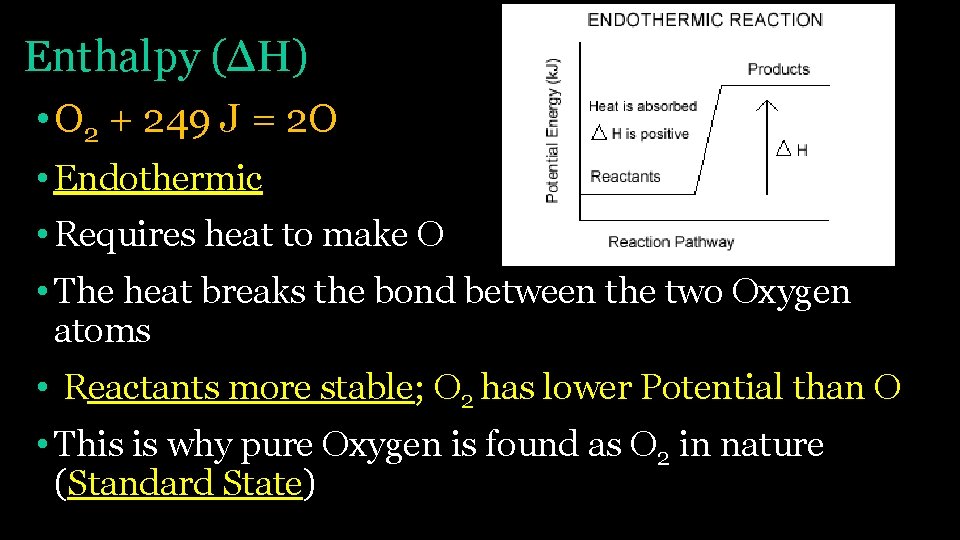
Enthalpy (ΔH) • O 2 + 249 J = 2 O • Endothermic • Requires heat to make O • The heat breaks the bond between the two Oxygen atoms • Reactants more stable; O 2 has lower Potential than O • This is why pure Oxygen is found as O 2 in nature (Standard State)