HEAT MEASURING TEMPERATURES Expansion an increase in the




- Slides: 12

HEAT

MEASURING TEMPERATURES: • Expansion = an increase in the volume of an object or substance; for most substances, adding heat causes it. • Contraction = a decrease in volume of an object or substance; usually with the removal of heat. • Since solids, liquids, and gases usually expand when heated and contract when cooled, almost any substance can be used in a thermometer. • Not all thermometers rely on expansion or contraction; some rely on electricity or battery power to operate. The choice usually depends on the way thermometer will be used.

MEASURING TEMPERATURES: Liquid Thermometers: • Use the expansion and contraction of a liquid to measure temp. • When warmed, the liquid (usually coloured ethyl alcohol) expands in the bulb and is forced up the narrow bore clinical thermometer outdoorthermometer

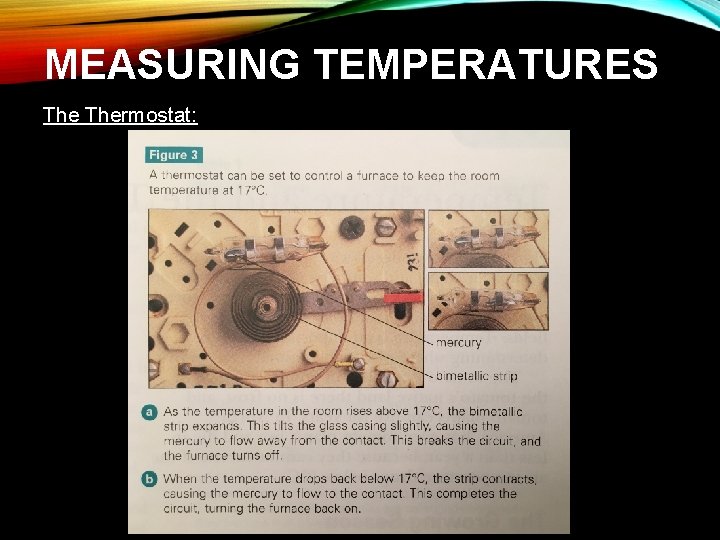
MEASURING TEMPERATURES: Thermostat: • Can be used to measure temperature in a room or in an appliance (such as a furnace) • It can also switch appliances on or off at a pre-set temperature • It can act as a feedback system • Use the expansion and contraction of solids to measure temp. • They contain a strip made of two metals (bimetallic strip) – because they are different substances, when they are heated or cooled, the two metals will expand or contract by different amounts causing the strip to bend • The amount of bending depends on the temperature

MEASURING TEMPERATURES: Thermostat:

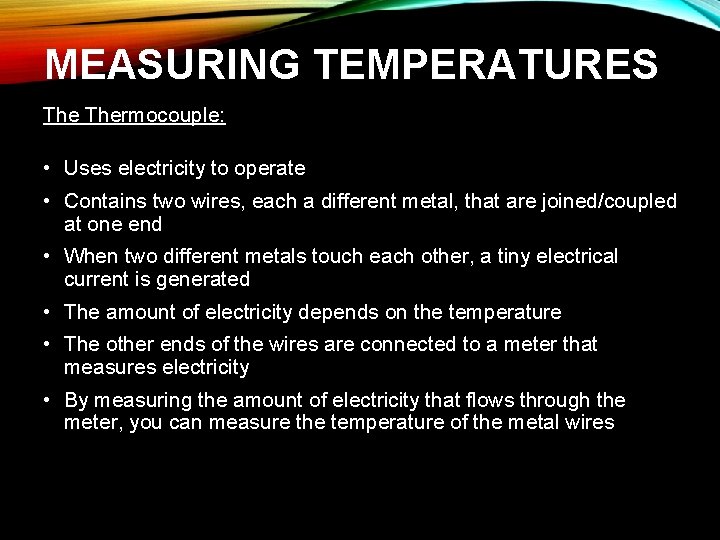
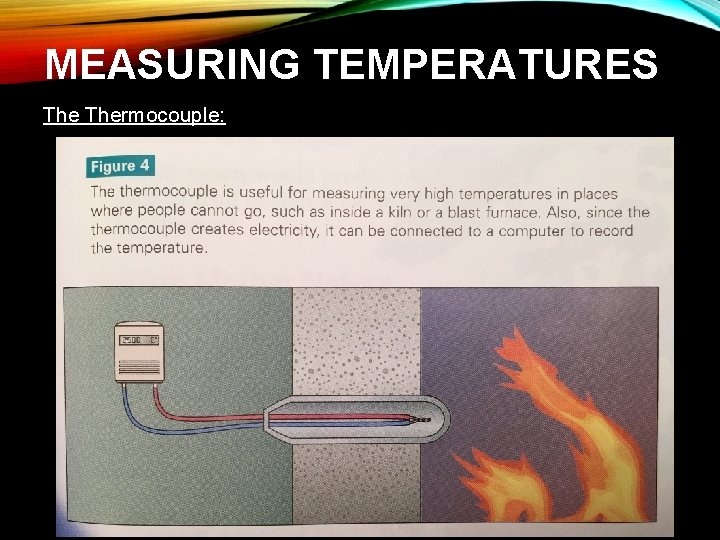
MEASURING TEMPERATURES: Thermocouple: • Uses electricity to operate • Contains two wires, each a different metal, that are joined/coupled at one end • When two different metals touch each other, a tiny electrical current is generated • The amount of electricity depends on the temperature • The other ends of the wires are connected to a meter that measures electricity • By measuring the amount of electricity that flows through the meter, you can measure the temperature of the metal wires

MEASURING TEMPERATURES: Thermocouple:

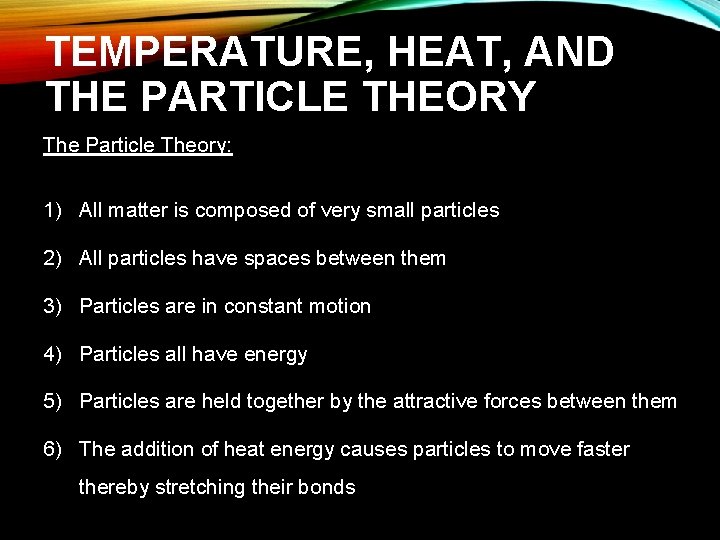
TEMPERATURE, HEAT, AND THE PARTICLE THEORY The Particle Theory: 1) All matter is composed of very small particles 2) All particles have spaces between them 3) Particles are in constant motion 4) Particles all have energy 5) Particles are held together by the attractive forces between them 6) The addition of heat energy causes particles to move faster thereby stretching their bonds

TEMPERATURE, HEAT, AND THE PARTICLE THEORY Heat and Temperature: • Heat = energy (transfers from hotter substances to colder ones) • Temperature = a measure of the average energy of motion of the particles in a substance. • When heat is transferred to particles of cold water, for example, → particles will have more energy of motion → particles will move faster → temperature of the water rises

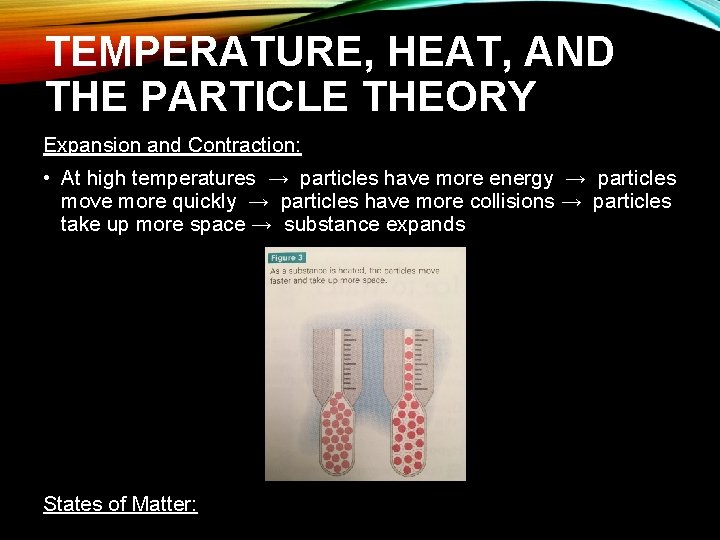
TEMPERATURE, HEAT, AND THE PARTICLE THEORY Expansion and Contraction: • At high temperatures → particles have more energy → particles move more quickly → particles have more collisions → particles take up more space → substance expands States of Matter:

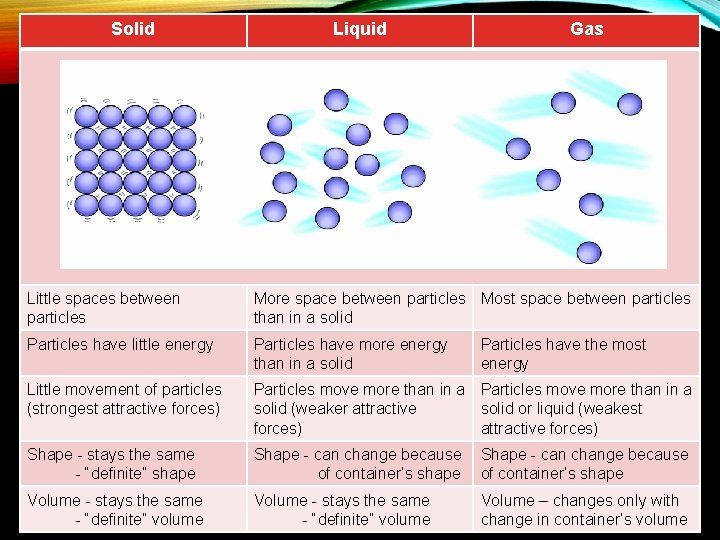
Solid Liquid Gas Little spaces between particles More space between particles Most space between particles than in a solid Particles have little energy Particles have more energy than in a solid Little movement of particles (strongest attractive forces) Particles move more than in a solid (weaker attractive solid or liquid (weakest forces) attractive forces) Shape - stays the same - “definite” shape Shape - can change because of container’s shape Volume - stays the same - “definite” volume Volume – changes only with change in container’s volume Particles have the most energy

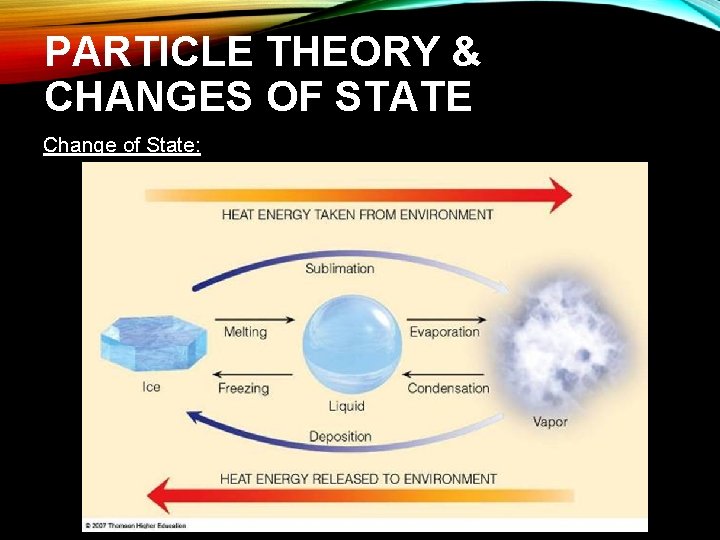
PARTICLE THEORY & CHANGES OF STATE Change of State: