Heat Engines Power Plants and Automotive power Lecture



- Slides: 12

Heat Engines: Power Plants and Automotive power Lecture 5 and Lecture 6 Prof. Chetan S. Solanki Department of Energy Science and Engineering, IIT Bombay E for Energy

Recap of last lecture E for Energy 1/16/2022 IIIT Bombay, Chetan. S. Solanki 2

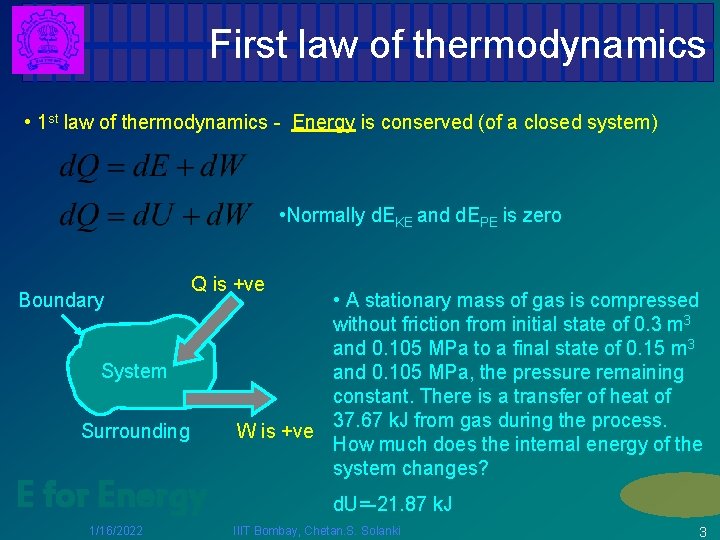
First law of thermodynamics • 1 st law of thermodynamics - Energy is conserved (of a closed system) • Normally d. EKE and d. EPE is zero Boundary Q is +ve System Surrounding E for Energy 1/16/2022 • A stationary mass of gas is compressed without friction from initial state of 0. 3 m 3 and 0. 105 MPa to a final state of 0. 15 m 3 and 0. 105 MPa, the pressure remaining constant. There is a transfer of heat of 37. 67 k. J from gas during the process. W is +ve How much does the internal energy of the system changes? d. U=-21. 87 k. J IIIT Bombay, Chetan. S. Solanki 3

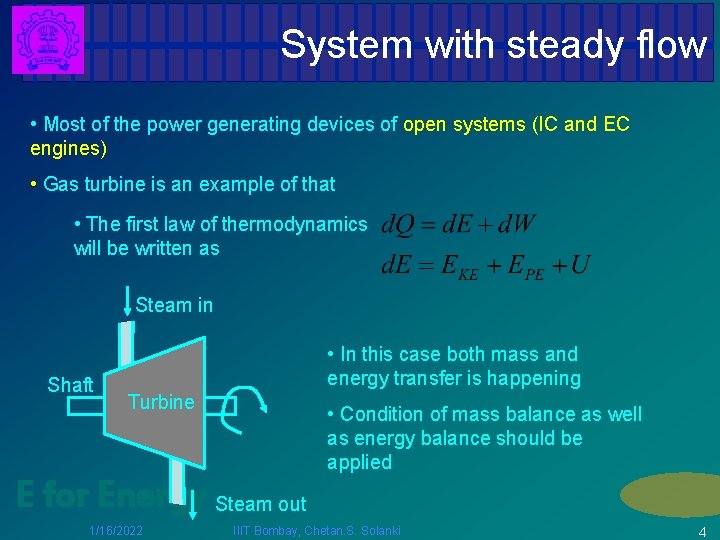
System with steady flow • Most of the power generating devices of open systems (IC and EC engines) • Gas turbine is an example of that • The first law of thermodynamics will be written as Steam in Shaft • In this case both mass and energy transfer is happening Turbine • Condition of mass balance as well as energy balance should be applied E for Energy Steam out 1/16/2022 IIIT Bombay, Chetan. S. Solanki 4

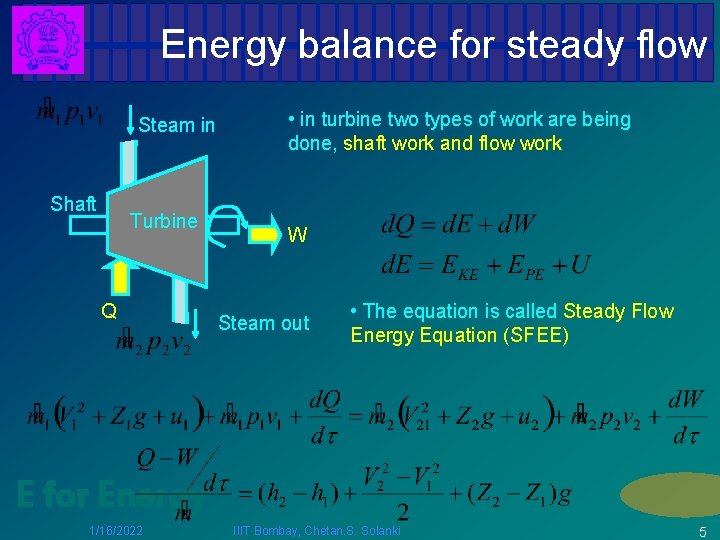
Energy balance for steady flow Steam in Shaft Turbine Q • in turbine two types of work are being done, shaft work and flow work W Steam out • The equation is called Steady Flow Energy Equation (SFEE) E for Energy 1/16/2022 IIIT Bombay, Chetan. S. Solanki 5

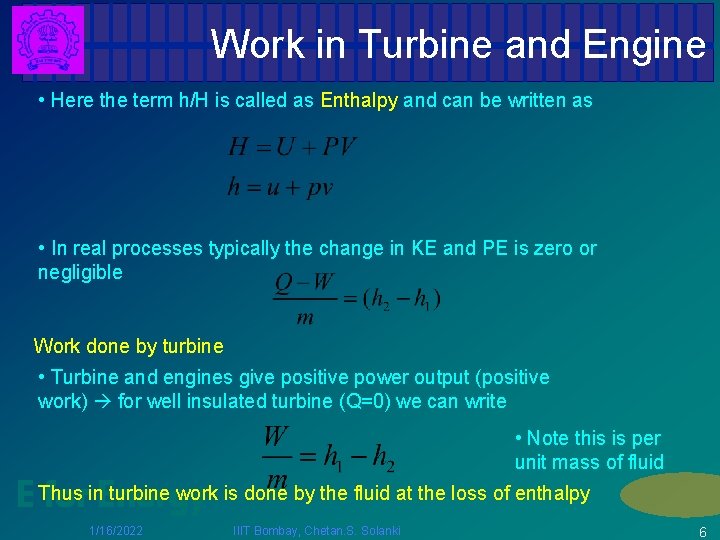
Work in Turbine and Engine • Here the term h/H is called as Enthalpy and can be written as • In real processes typically the change in KE and PE is zero or negligible Work done by turbine • Turbine and engines give positive power output (positive work) for well insulated turbine (Q=0) we can write • Note this is per unit mass of fluid turbine work is done by the fluid at the loss of enthalpy E Thus forin. Energy 1/16/2022 IIIT Bombay, Chetan. S. Solanki 6

Work in compressor and pump Work done by compressor or pump • Pump and compressor give negative power output (negative work) for well insulated pump (Q=0) we can write Thus in pump work is done on the fluid which results in increase in its enthalpy E for Energy 1/16/2022 IIIT Bombay, Chetan. S. Solanki 7

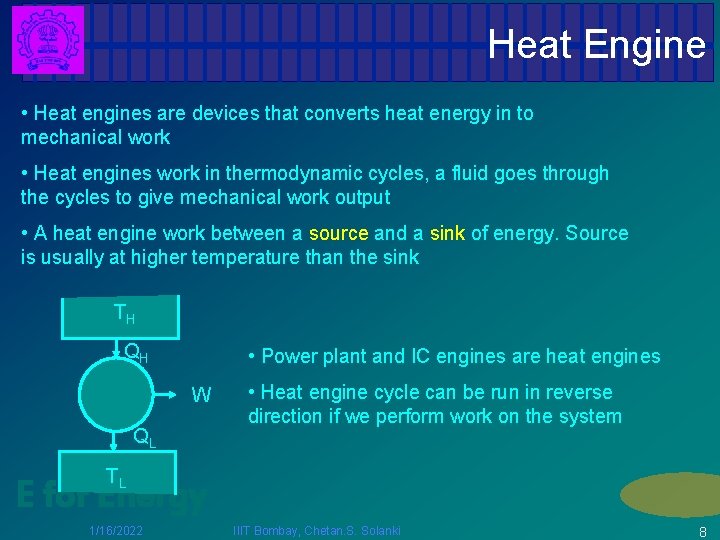
Heat Engine • Heat engines are devices that converts heat energy in to mechanical work • Heat engines work in thermodynamic cycles, a fluid goes through the cycles to give mechanical work output • A heat engine work between a source and a sink of energy. Source is usually at higher temperature than the sink TH QH • Power plant and IC engines are heat engines W QL • Heat engine cycle can be run in reverse direction if we perform work on the system TL E for Energy 1/16/2022 IIIT Bombay, Chetan. S. Solanki 8

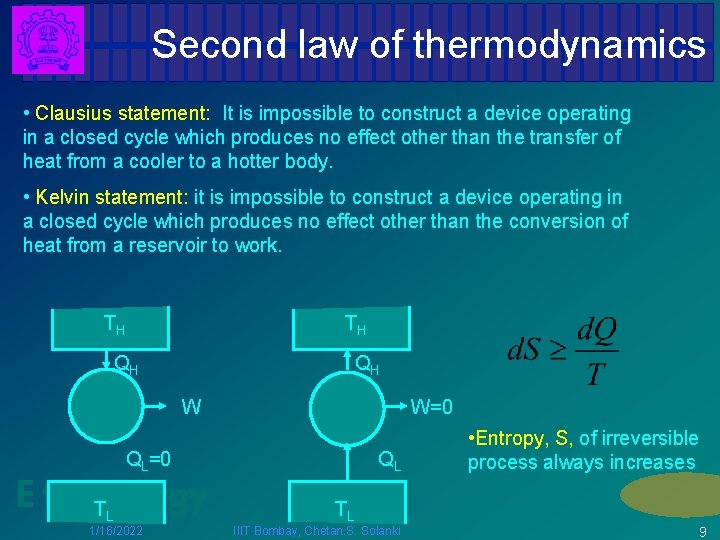
Second law of thermodynamics • Clausius statement: It is impossible to construct a device operating in a closed cycle which produces no effect other than the transfer of heat from a cooler to a hotter body. • Kelvin statement: it is impossible to construct a device operating in a closed cycle which produces no effect other than the conversion of heat from a reservoir to work. TH TH QH QH W W=0 QL=0 E for TEnergy L 1/16/2022 QL • Entropy, S, of irreversible process always increases TL IIIT Bombay, Chetan. S. Solanki 9

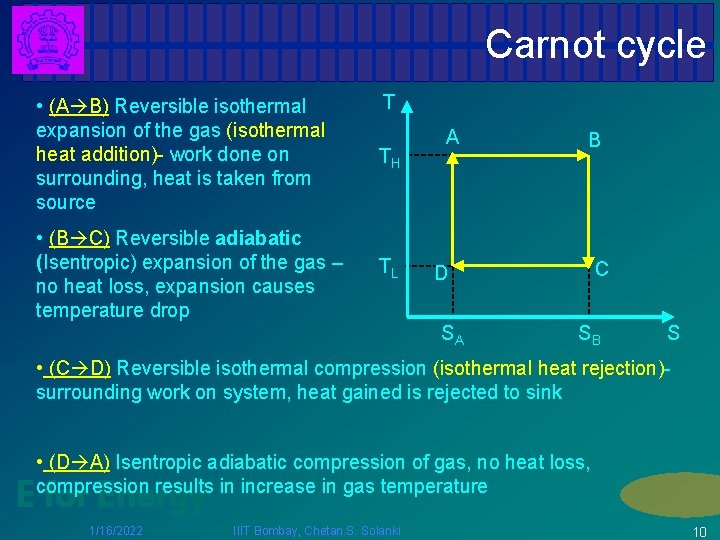
Carnot cycle T • (A B) Reversible isothermal expansion of the gas (isothermal heat addition)- work done on surrounding, heat is taken from source TH • (B C) Reversible adiabatic (Isentropic) expansion of the gas – no heat loss, expansion causes temperature drop TL A B C D SA SB S • (C D) Reversible isothermal compression (isothermal heat rejection)surrounding work on system, heat gained is rejected to sink • (D A) Isentropic adiabatic compression of gas, no heat loss, compression results in increase in gas temperature E for Energy 1/16/2022 IIIT Bombay, Chetan. S. Solanki 10

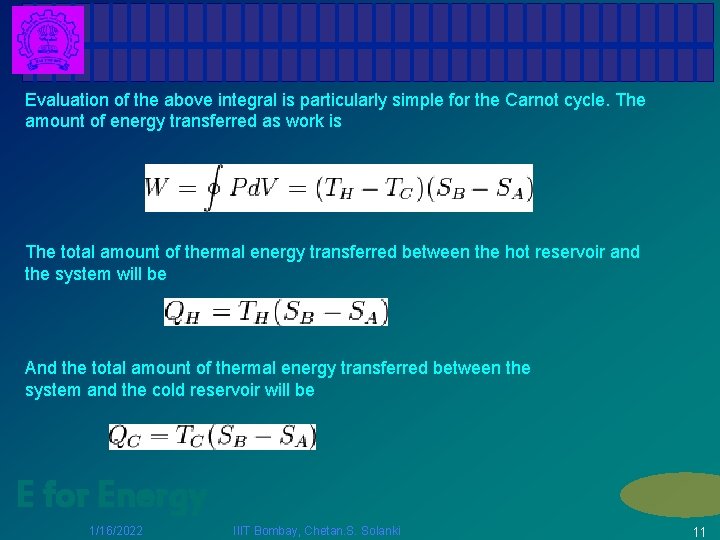
Evaluation of the above integral is particularly simple for the Carnot cycle. The amount of energy transferred as work is The total amount of thermal energy transferred between the hot reservoir and the system will be And the total amount of thermal energy transferred between the system and the cold reservoir will be E for Energy 1/16/2022 IIIT Bombay, Chetan. S. Solanki 11

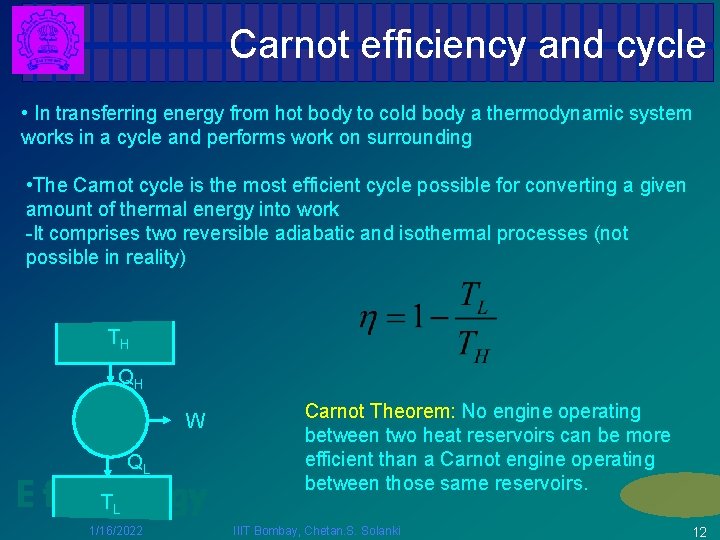
Carnot efficiency and cycle • In transferring energy from hot body to cold body a thermodynamic system works in a cycle and performs work on surrounding • The Carnot cycle is the most efficient cycle possible for converting a given amount of thermal energy into work -It comprises two reversible adiabatic and isothermal processes (not possible in reality) TH QH W QL E for Energy T Carnot Theorem: No engine operating between two heat reservoirs can be more efficient than a Carnot engine operating between those same reservoirs. L 1/16/2022 IIIT Bombay, Chetan. S. Solanki 12