Heat Engines A heat engine is a system
- Slides: 8

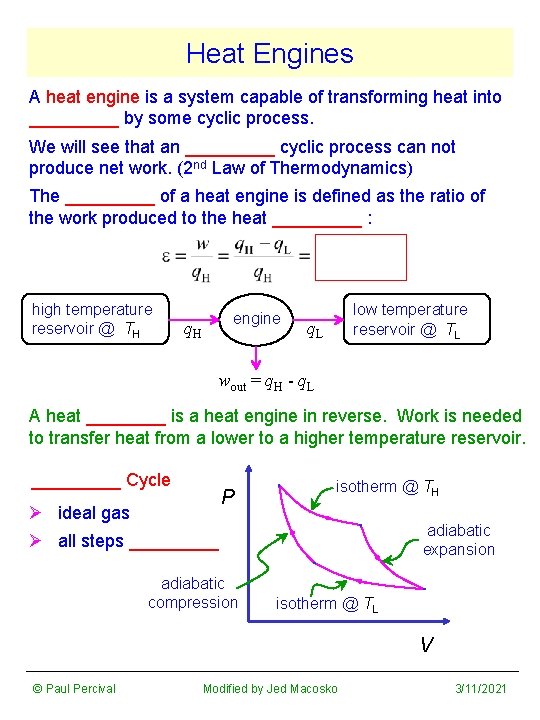
Heat Engines A heat engine is a system capable of transforming heat into _____ by some cyclic process. We will see that an _____ cyclic process can not produce net work. (2 nd Law of Thermodynamics) The _____ of a heat engine is defined as the ratio of the work produced to the heat _____ : high temperature reservoir @ TH engine q. H low temperature reservoir @ TL q. L wout = q. H - q. L A heat ____ is a heat engine in reverse. Work is needed to transfer heat from a lower to a higher temperature reservoir. _____ Cycle P Ø ideal gas isotherm @ TH adiabatic expansion Ø all steps _____ adiabatic compression isotherm @ TL V © Paul Percival Modified by Jed Macosko 3/11/2021

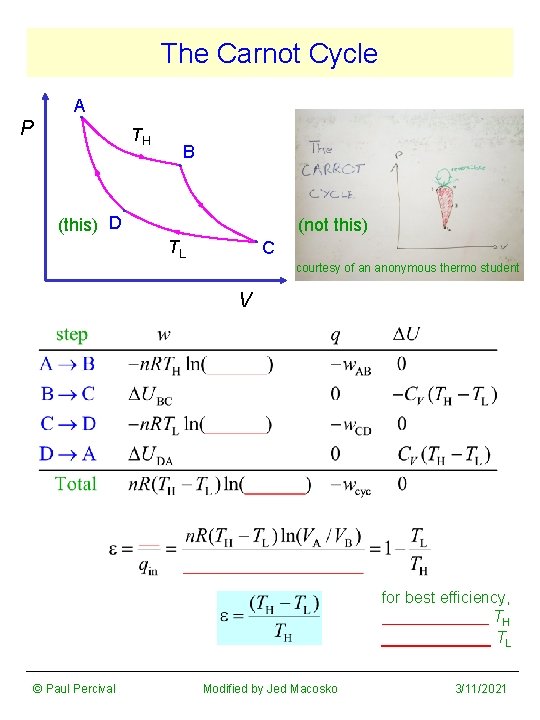
The Carnot Cycle A P TH B (this) D (not this) TL C courtesy of an anonymous thermo student V for best efficiency, ______ TH ______ TL © Paul Percival Modified by Jed Macosko 3/11/2021

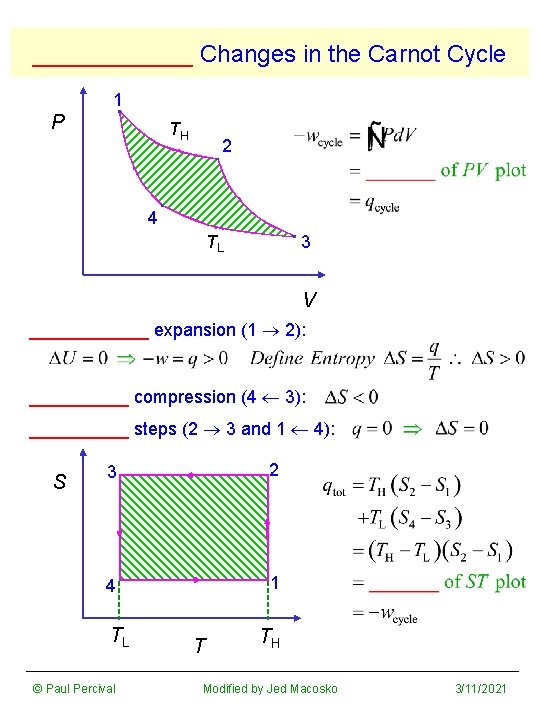
______ Changes in the Carnot Cycle 1 P TH 2 4 TL 3 V ______ expansion (1 2): _____ compression (4 3): _____ steps (2 3 and 1 4): S 3 2 4 1 TL © Paul Percival T TH Modified by Jed Macosko 3/11/2021

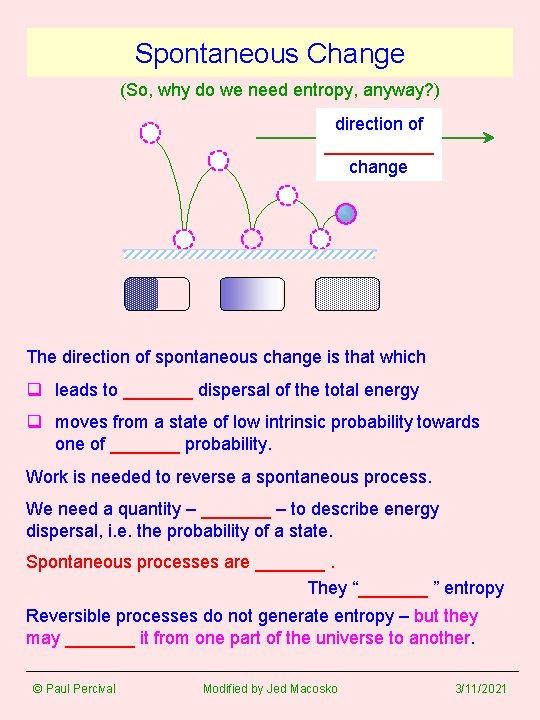
Spontaneous Change (So, why do we need entropy, anyway? ) direction of ______ change The direction of spontaneous change is that which q leads to _______ dispersal of the total energy q moves from a state of low intrinsic probability towards one of _______ probability. Work is needed to reverse a spontaneous process. We need a quantity – _______ – to describe energy dispersal, i. e. the probability of a state. Spontaneous processes are _______. They “_______ ” entropy Reversible processes do not generate entropy – but they may _______ it from one part of the universe to another. © Paul Percival Modified by Jed Macosko 3/11/2021

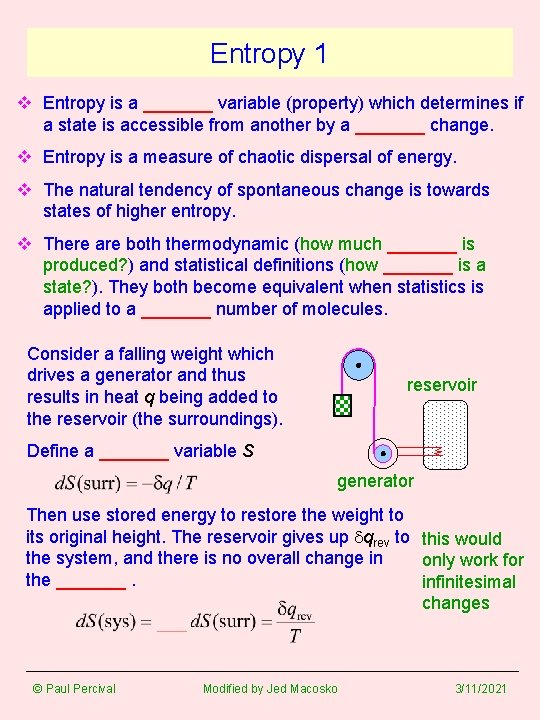
Entropy 1 v Entropy is a _______ variable (property) which determines if a state is accessible from another by a _______ change. v Entropy is a measure of chaotic dispersal of energy. v The natural tendency of spontaneous change is towards states of higher entropy. v There are both thermodynamic (how much _______ is produced? ) and statistical definitions (how _______ is a state? ). They both become equivalent when statistics is applied to a _______ number of molecules. Consider a falling weight which drives a generator and thus results in heat q being added to the reservoir (the surroundings). reservoir Define a _______ variable S generator Then use stored energy to restore the weight to its original height. The reservoir gives up dqrev to this would the system, and there is no overall change in only work for the _______. infinitesimal changes © Paul Percival Modified by Jed Macosko 3/11/2021

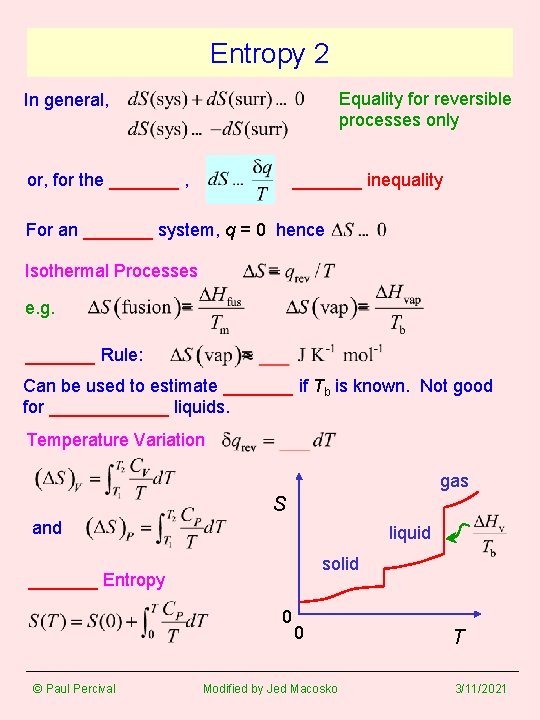
Entropy 2 Equality for reversible processes only In general, or, for the _______ , _______ inequality For an _______ system, q = 0 hence Isothermal Processes e. g. _______ Rule: Can be used to estimate _______ if Tb is known. Not good for ______ liquids. Temperature Variation gas S and liquid solid _______ Entropy 0 © Paul Percival 0 Modified by Jed Macosko T 3/11/2021

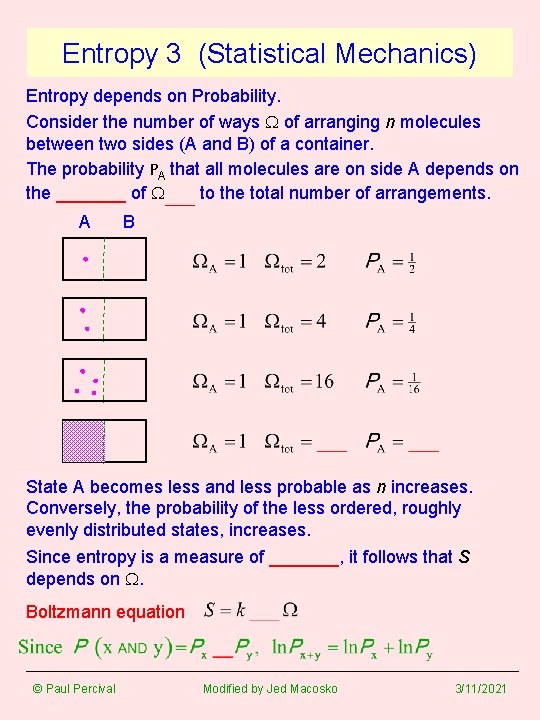
Entropy 3 (Statistical Mechanics) Entropy depends on Probability. Consider the number of ways W of arranging n molecules between two sides (A and B) of a container. The probability PA that all molecules are on side A depends on the _______ of W_____ to the total number of arrangements. A B State A becomes less and less probable as n increases. Conversely, the probability of the less ordered, roughly evenly distributed states, increases. Since entropy is a measure of _______, it follows that S depends on W. Boltzmann equation © Paul Percival Modified by Jed Macosko 3/11/2021

The _______ Law of Thermodynamics Ø “An _______ cyclic process in which there is a net conversion of _______ into work is impossible. ” Ø “No process is possible in which the sole result is the absorption of heat from a reservoir and its conversion into work. ” It is possible to convert _______ work into heat! Ø “It is impossible for heat to be transformed from a body at a lower temperature to one at a higher temperature unless _______ is done. ” Ø “The entropy of an isolated system _______ during any natural process. ” The universe is an isolated system. DS(sys) < 0 is allowed provided DS(sys) + DS(surr) > 0 Ø “All reversible _______ cycles operating between the same two temperatures have the same thermodynamic efficiency. ” Ø “There is a state function called entropy S that can be calculated from __S = dqrev/T. The change in entropy in any process is given by d. S dq/T, where the inequality refers to a spontaneous (irreversible) process. ” The 1 st Law uses U to identify _______ changes of state. The 2 nd Law uses S to identify _______ changes among the permissible ones. © Paul Percival Modified by Jed Macosko 3/11/2021