Heat Energy Heat thermal energy is simply a





- Slides: 14

Heat Energy: ¡ Heat (thermal) energy is simply a type of energy l l ¡ What is heat energy? Ex. heat from a stove heat from a light bulb Heat energy can be transferred from one object to another l Ex. from a hot coffee cup to your cold hands

Heat Energy ¡ It is possible to calculate the amount of heat energy absorbed or released by a given substance using the following formula:

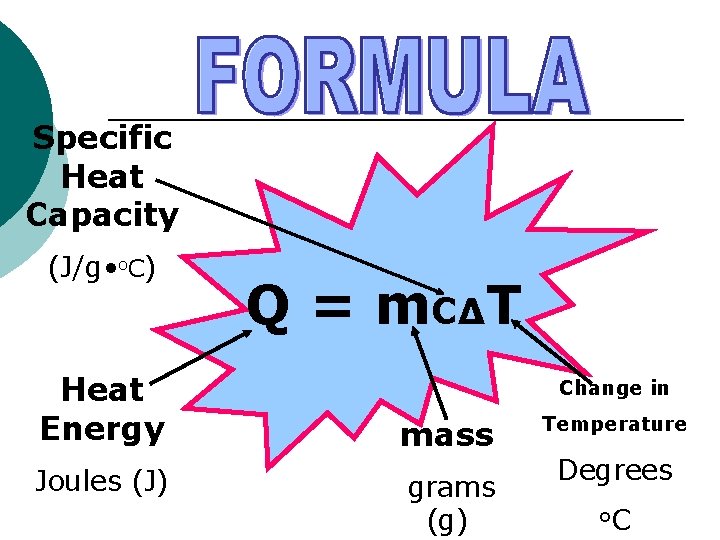
Specific Heat Capacity (J/g • o. C) Heat Energy Joules (J) Q = m. C∆T Change in mass grams (g) Temperature Degrees o. C

Specific Capacity (c) specific heat capacity (c) - the amount of energy required to heat up or cool down a substance. It is a characteristic property. Every substance has their own specific heat capacity that does not change. However, water is most commonly used in exam questions. Substances Joules/(g • 0 C) Copper 0. 383 Iron 0. 452 Aluminum 0. 896 Antifreeze 2. 2 Methanol 2. 547 Water 4. 19

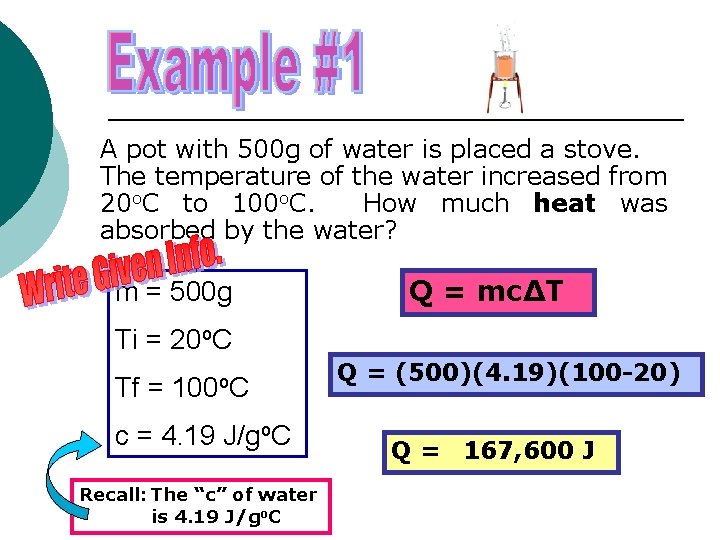
A pot with 500 g of water is placed a stove. The temperature of the water increased from 20 o. C to 100 o. C. How much heat was absorbed by the water? m = 500 g Q = mc∆T Ti = 20 o. C Tf = 100 o. C c = 4. 19 J/go. C Recall: The “c” of water is 4. 19 J/go. C Q = (500)(4. 19)(100 -20) Q = 167, 600 J

A 250 g glass of cold ice water is placed outside in the sun. The temperature of the water rises from 5 o. C to 25 o. C. How much heat was absorbed by the water? m = 250 g Q = mc∆T Ti = 5 o. C Tf = 25 o. C c = 4. 19 J/go. C Q = (250)(4. 19)(25 -5) Q = 20, 950 J

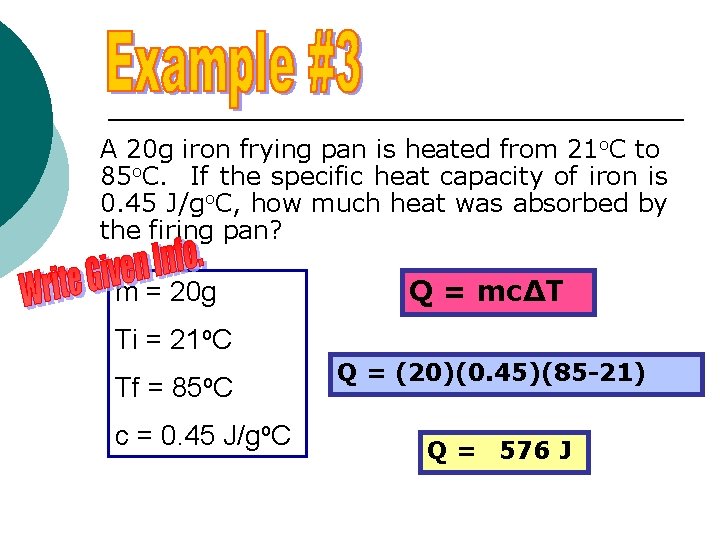
A 20 g iron frying pan is heated from 21 o. C to 85 o. C. If the specific heat capacity of iron is 0. 45 J/go. C, how much heat was absorbed by the firing pan? m = 20 g Q = mc∆T Ti = 21 o. C Tf = 85 o. C c = 0. 45 J/go. C Q = (20)(0. 45)(85 -21) Q = 576 J

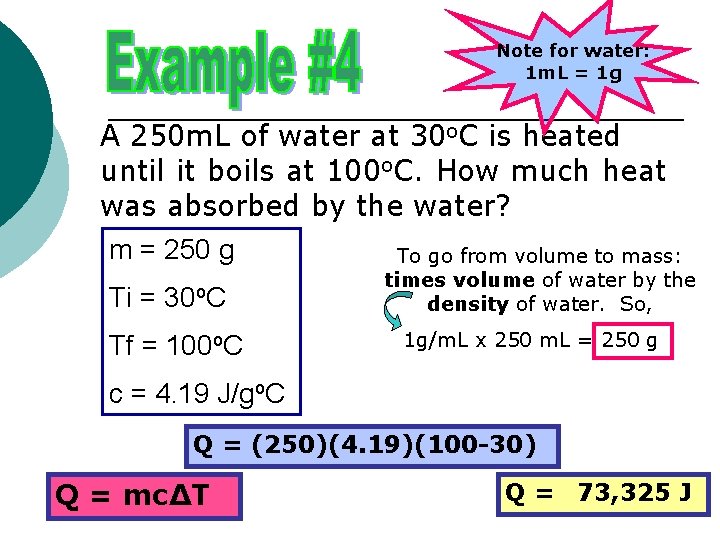
Note for water: 1 m. L = 1 g A 250 m. L of water at 30 o. C is heated until it boils at 100 o. C. How much heat was absorbed by the water? m = 250 g To go from volume to mass: Ti = 30 o. C Tf = 100 o. C times volume of water by the density of water. So, 1 g/m. L x 250 m. L = 250 g c = 4. 19 J/go. C Q = (250)(4. 19)(100 -30) Q = mc∆T Q = 73, 325 J

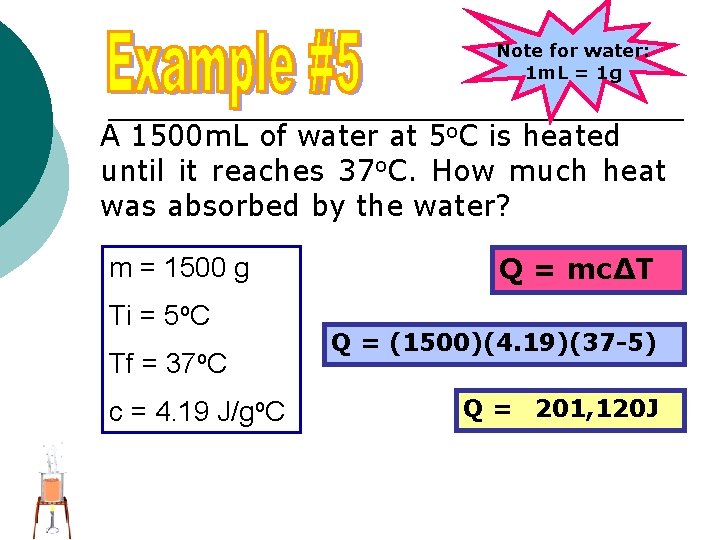
Note for water: 1 m. L = 1 g A 1500 m. L of water at 5 o. C is heated until it reaches 37 o. C. How much heat was absorbed by the water? m = 1500 g Ti = 5 o. C Tf = 37 o. C c = 4. 19 J/go. C Q = mc∆T Q = (1500)(4. 19)(37 -5) Q = 201, 120 J

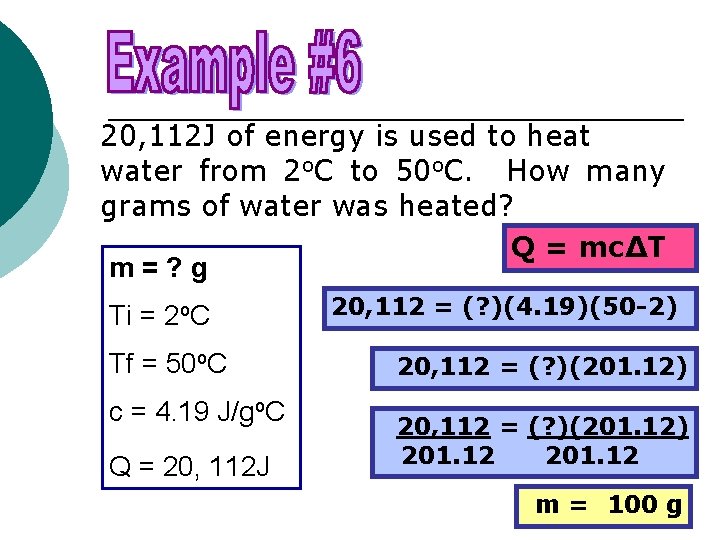
20, 112 J of energy is used to heat water from 2 o. C to 50 o. C. How many grams of water was heated? Q = mc∆T m=? g Ti = 2 o. C Tf = 50 o. C c = 4. 19 J/go. C Q = 20, 112 J 20, 112 = (? )(4. 19)(50 -2) 20, 112 = (? )(201. 12) 201. 12 m = 100 g

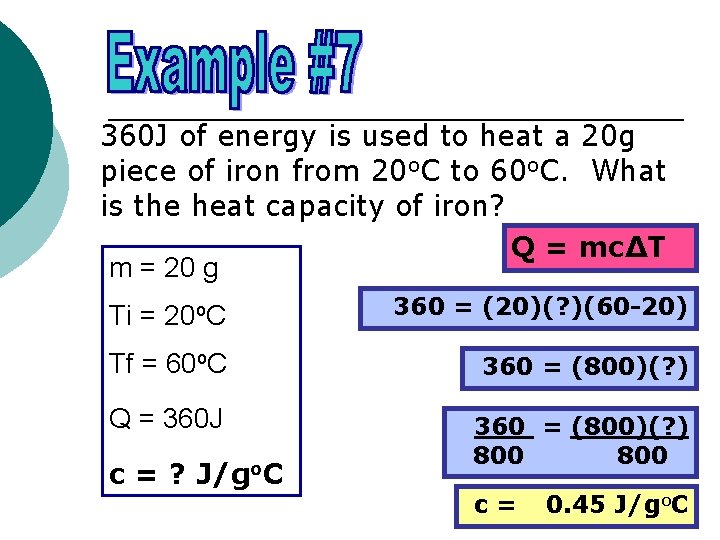
360 J of energy is used to heat a 20 g piece of iron from 20 o. C to 60 o. C. What is the heat capacity of iron? Q = mc∆T m = 20 g Ti = 20 o. C 360 = (20)(? )(60 -20) Tf = 60 o. C 360 = (800)(? ) Q = 360 J 360 = (800)(? ) 800 c = ? J/go. C c= 0. 45 J/go. C

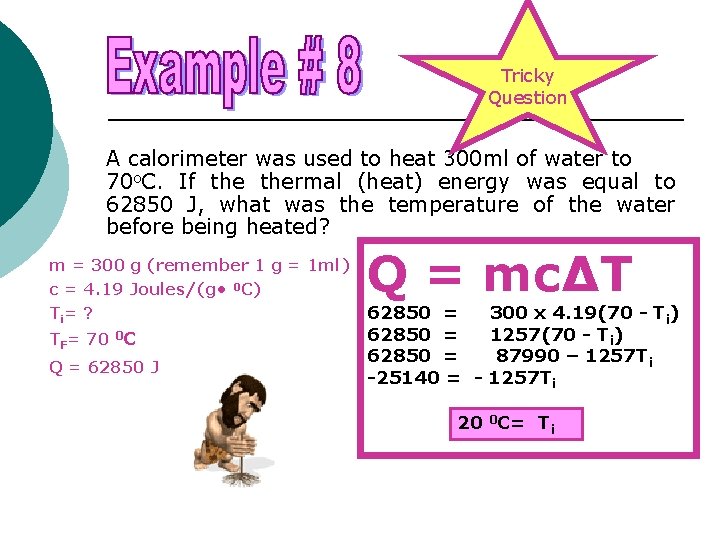
Tricky Question A calorimeter was used to heat 300 ml of water to 70 o. C. If thermal (heat) energy was equal to 62850 J, what was the temperature of the water before being heated? m = 300 g (remember 1 g = 1 ml) c = 4. 19 Joules/(g • 0 C) Ti = ? TF= 70 0 C Q = 62850 J Q = mc∆T 62850 = -25140 = - 300 x 4. 19(70 - Ti) 1257(70 - Ti) 87990 – 1257 Ti 20 0 C= Ti

Tricky Question A calorimeter was used to heat 400 ml of water to 40 o. C. If thermal (heat) energy was equal to 5. 4 k. J, what was the initial temperature of the water before being heated? m = 400 g (remember 1 g = 1 ml) c = 4. 19 Joules/(g • 0 C) Ti = ? TF= 40 0 C Q = 5400 (5. 4 k. J = 5400 J) Q = mc∆T 5400 = 400 x 4. 19(40 - Ti) 5400 = 1676(40 - Ti) 5400 = 67040 – 1676 Ti -61640 = - 1676 Ti 36. 8= Ti

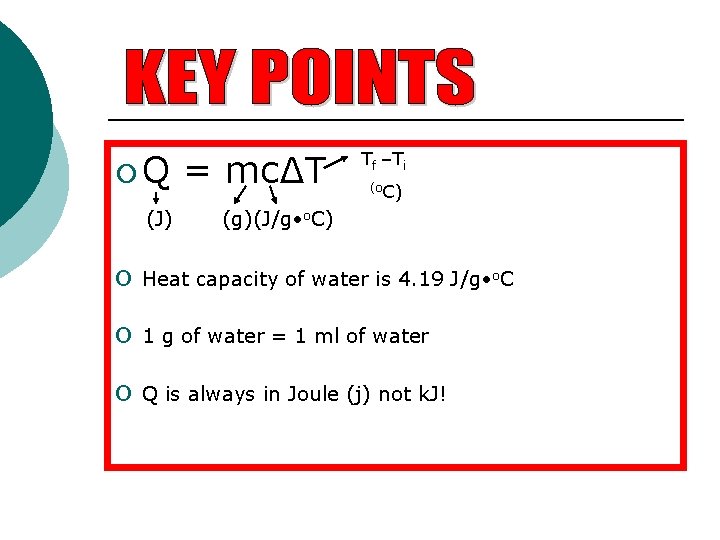
¡Q (J) = mc∆T Tf –Ti (o. C) (g)(J/g • o. C) o Heat capacity of water is 4. 19 J/g • o. C o 1 g of water = 1 ml of water o Q is always in Joule (j) not k. J!
 Thermal energy section 3
Thermal energy section 3 How are thermal energy and temperature different
How are thermal energy and temperature different Conceptual physical science 5th edition
Conceptual physical science 5th edition Difference between heat and thermal energy
Difference between heat and thermal energy Specific heat capacity of lead j/kg c
Specific heat capacity of lead j/kg c Difference between heat and thermal energy
Difference between heat and thermal energy Heat vs thermal energy vs temperature
Heat vs thermal energy vs temperature What is the difference between thermal energy and heat?
What is the difference between thermal energy and heat? Energy and temperature
Energy and temperature Heat transfer types
Heat transfer types Heat thermal energy and temperature
Heat thermal energy and temperature Thermal vs heat energy
Thermal vs heat energy Heat flow
Heat flow Heat vs thermal energy vs temperature
Heat vs thermal energy vs temperature Q mct
Q mct