HEAT Conversion Temperatures Temperature Scales n Fahrenheit n





- Slides: 45

HEAT Conversion. Temperatures:

Temperature Scales n Fahrenheit n Celsius n Kelvin

Helpful Hints n Identify the equation needed. n Plug in the numbers to solve n Remember the math rules: • Solve what is in parenthesis first • Solve Multiplication & Division before addition and subtraction n Show all work n Put box around final answer

Solving 2–Step temperature equations n Necessary Ø K to o. F Ø o. F to K only when converting:

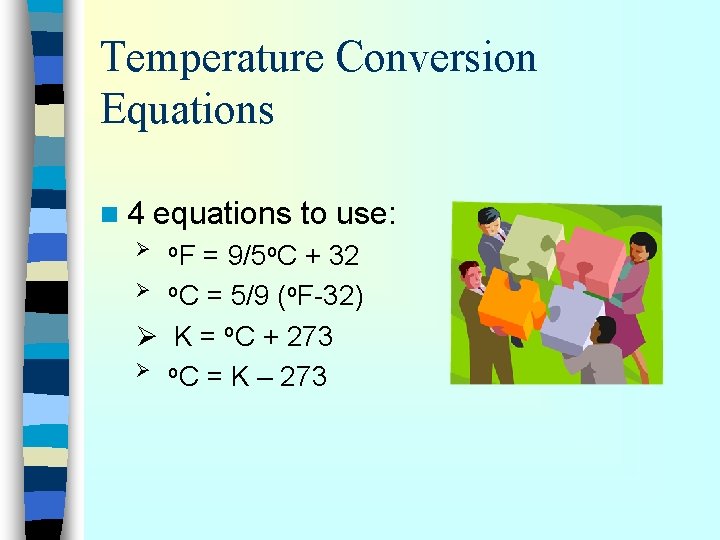
Temperature Conversion Equations n 4 equations to use: Ø o. F = 9/5 o. C + 32 Ø o. C = 5/9 (o. F-32) Ø K = o. C + 273 Ø o. C = K – 273

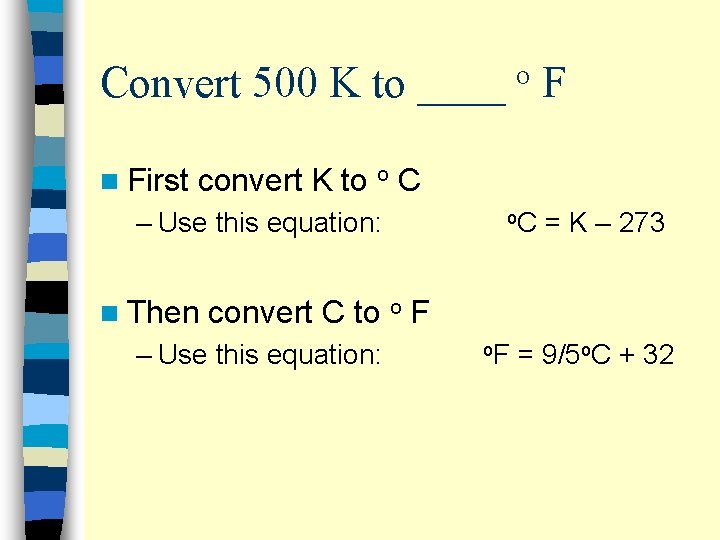
Convert 500 K to ____ F o n First convert K to o C – Use this equation: n Then o. C = K – 273 convert C to o F – Use this equation: o. F = 9/5 o. C + 32

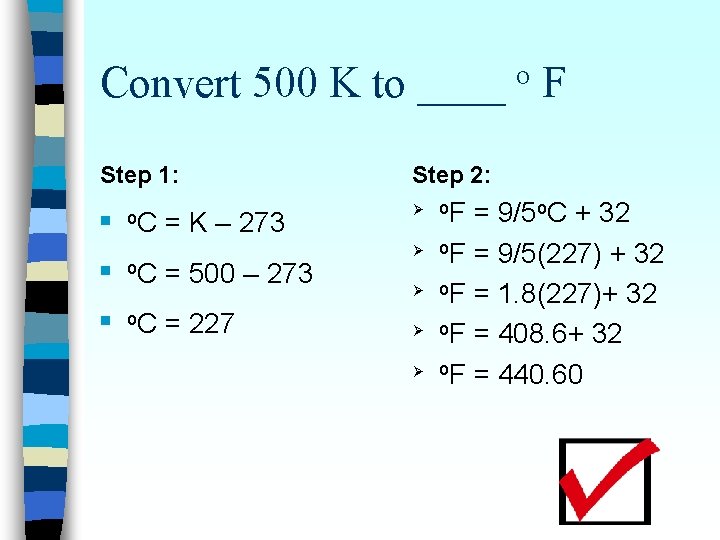
Convert 500 K to ____ F o Step 1: n n n o. C = K – 273 o. C = 500 – 273 o. C = 227 Step 2: Ø Ø Ø o. F = 9/5 o. C + 32 o. F = 9/5(227) + 32 o. F = 1. 8(227)+ 32 o. F = 408. 6+ 32 o. F = 440. 60

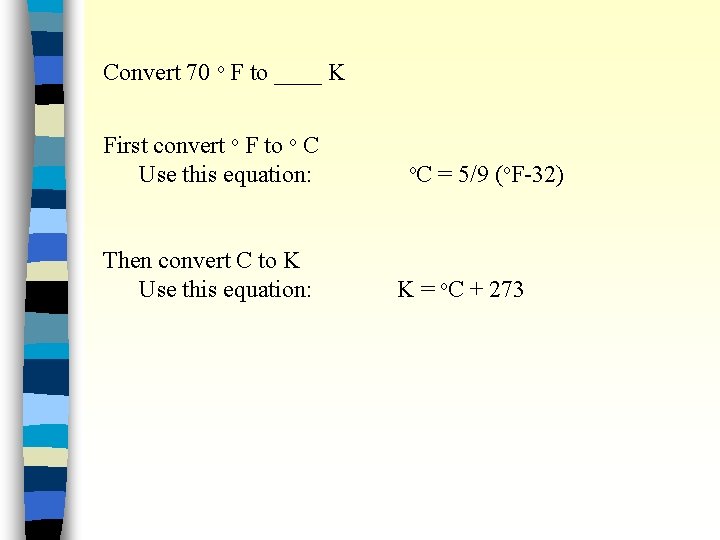
Convert 70 o F to ____ K First convert o F to o C Use this equation: Then convert C to K Use this equation: o. C = 5/9 (o. F-32) K = o. C + 273

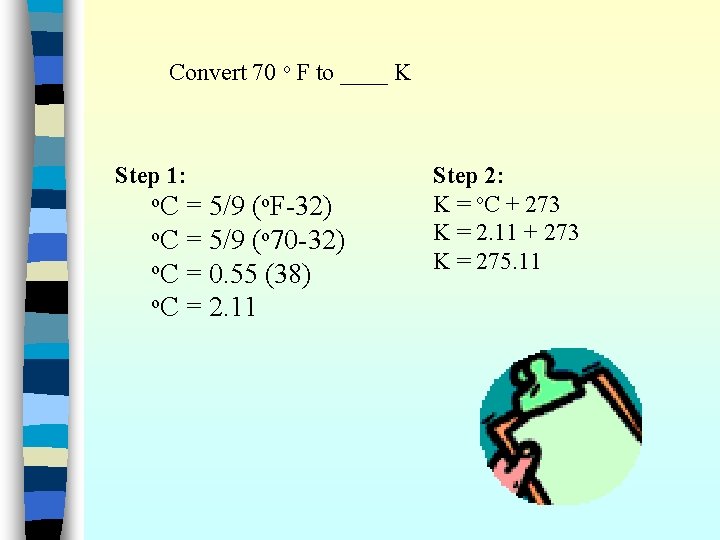
Convert 70 o F to ____ K Step 1: o. C = 5/9 (o. F-32) o. C = 5/9 (o 70 -32) o. C = 0. 55 (38) o. C = 2. 11 Step 2: K = o. C + 273 K = 2. 11 + 273 K = 275. 11

Units of Heat Objectives are to: n define and distinguish between various units of heat n define the mechanical equivalent of heat n discuss everyday examples to illustrate these concepts Temperature Conversions:

Units of Heat

Units of Heat n n n Heat is energy in transit, and is measured in energy units. The SI unit is the joule (J), or Newton-metre (Nm). Historically, heat was measured in terms of the ability to raise the temperature of water. The kilocalorie (kcal), or Calorie (Cal), or “big calorie”: amount of heat needed to raise the temperature of 1 kilogramme of water by 1 C 0 (from 14. 50 C to 15. 50 C) The calorie, or “little calorie”: amount of heat needed to raise the temperature of 1 gramme of water by 1 C 0 (from 14. 50 C to 15. 50 C) In industry, the British thermal unit (Btu) is still used: amount of heat needed to raise the temperature of 1 lb of water by 1 F 0 (from 630 F to 640 F)

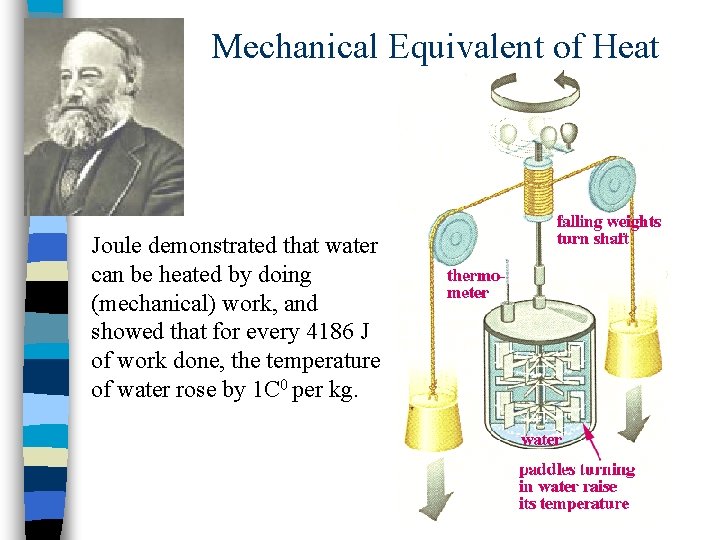
Mechanical Equivalent of Heat Joule demonstrated that water can be heated by doing (mechanical) work, and showed that for every 4186 J of work done, the temperature of water rose by 1 C 0 per kg.

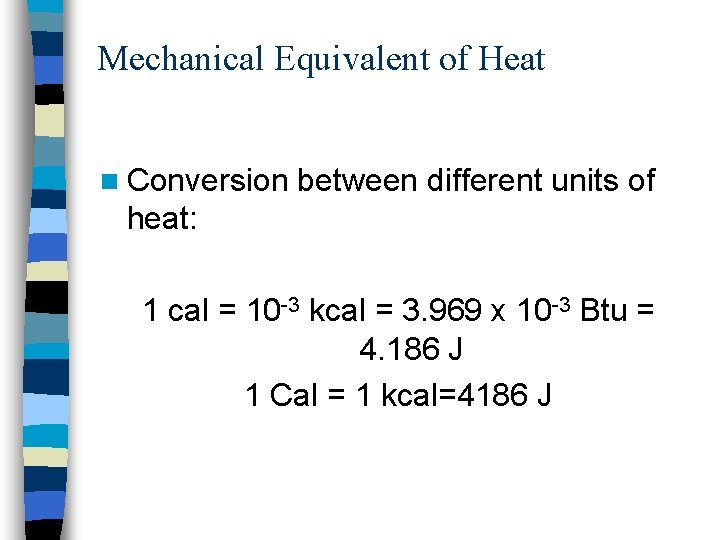
Mechanical Equivalent of Heat n Conversion between different units of heat: 1 cal = 10 -3 kcal = 3. 969 x 10 -3 Btu = 4. 186 J 1 Cal = 1 kcal=4186 J

Sensible Heat Objectives are to: n describe what is meant by 'sensible heat‘ n define specific heat n explain how the specific heat capacities of materials are obtained using calorimetry

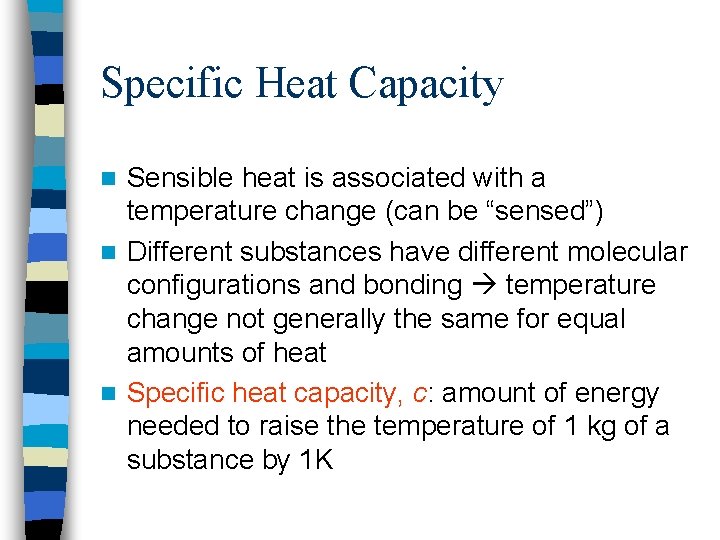
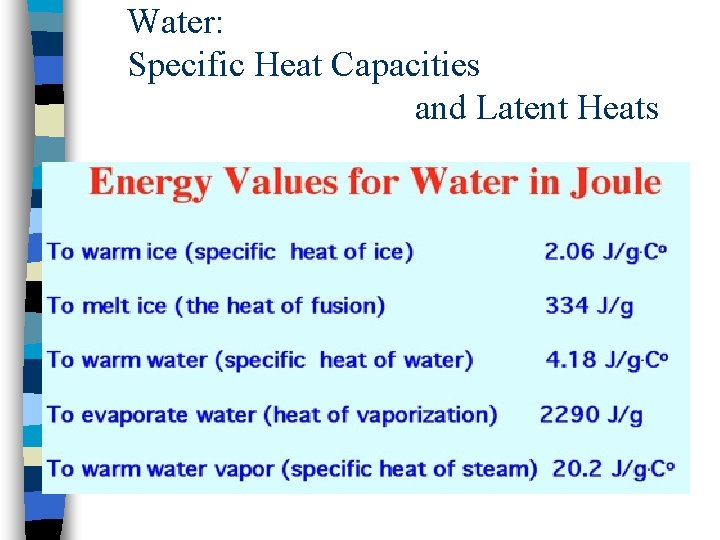
Specific Heat Capacity Sensible heat is associated with a temperature change (can be “sensed”) n Different substances have different molecular configurations and bonding temperature change not generally the same for equal amounts of heat n Specific heat capacity, c: amount of energy needed to raise the temperature of 1 kg of a substance by 1 K n

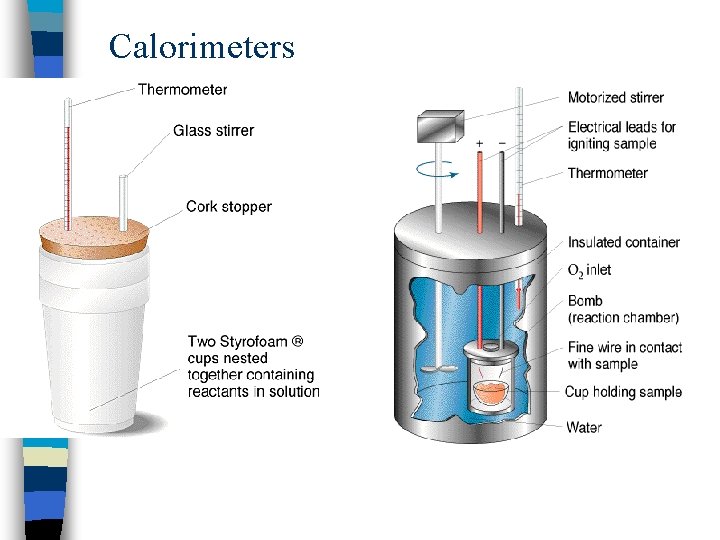
Calorimeters

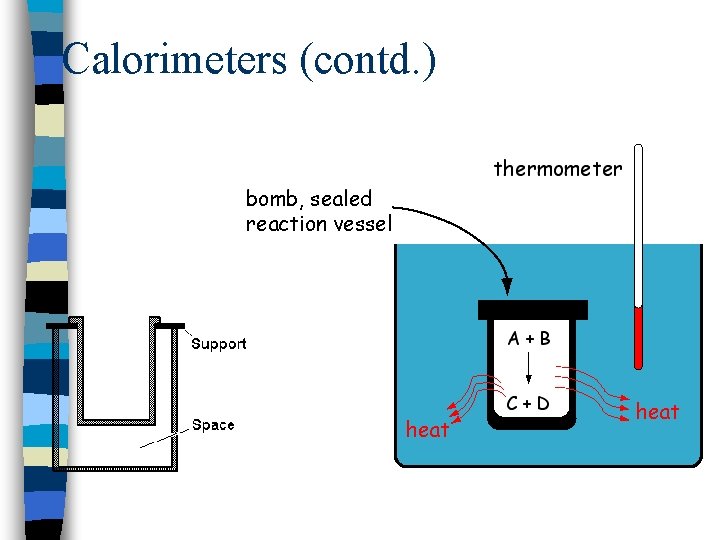
Calorimeters (contd. )

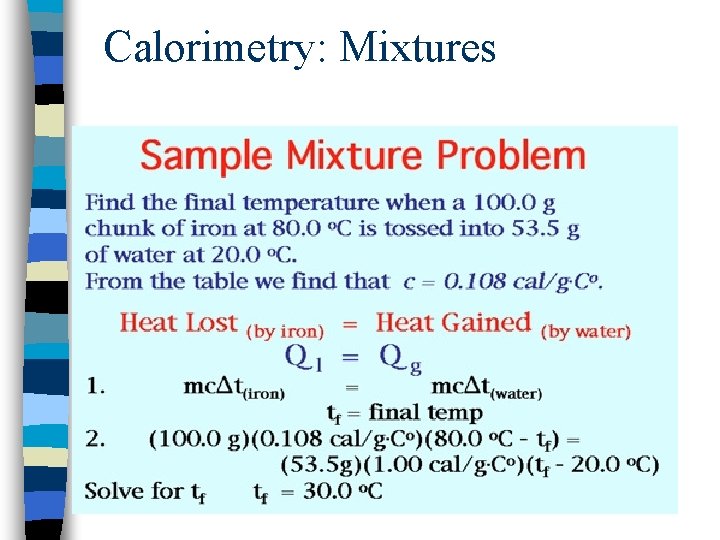
Calorimetry: An Exercise in Bookkeeping

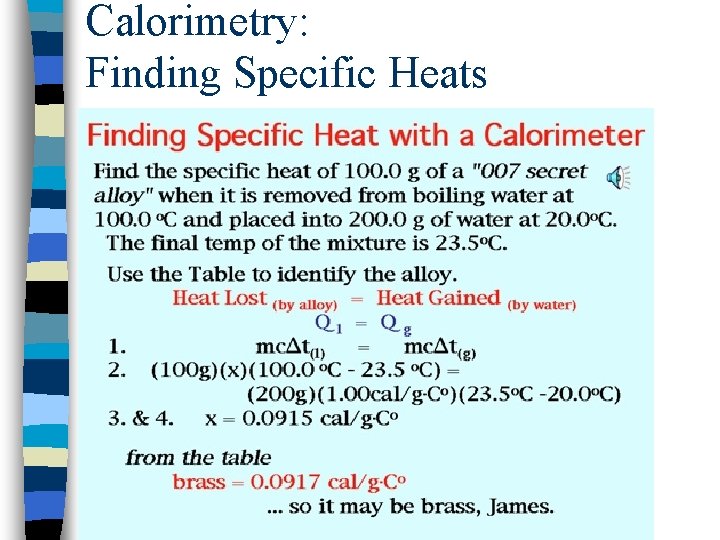
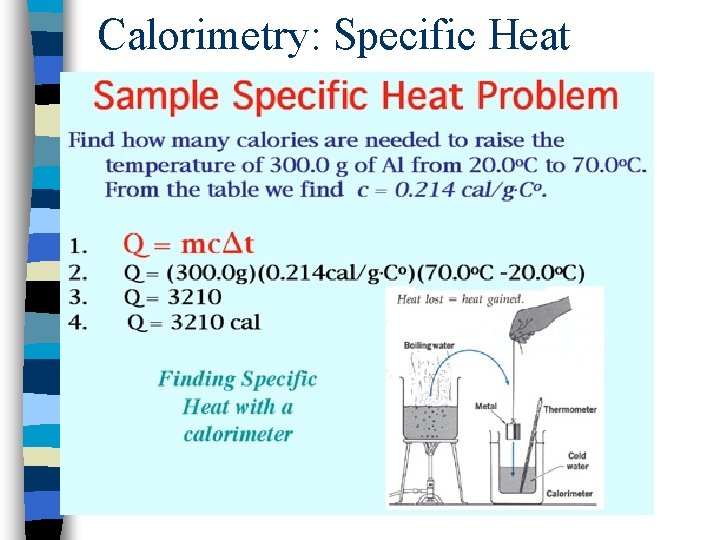
Calorimetry: Finding Specific Heats

Calorimetry: Specific Heat

Calorimetry: Mixtures

Water: Specific Heat Capacities and Latent Heats

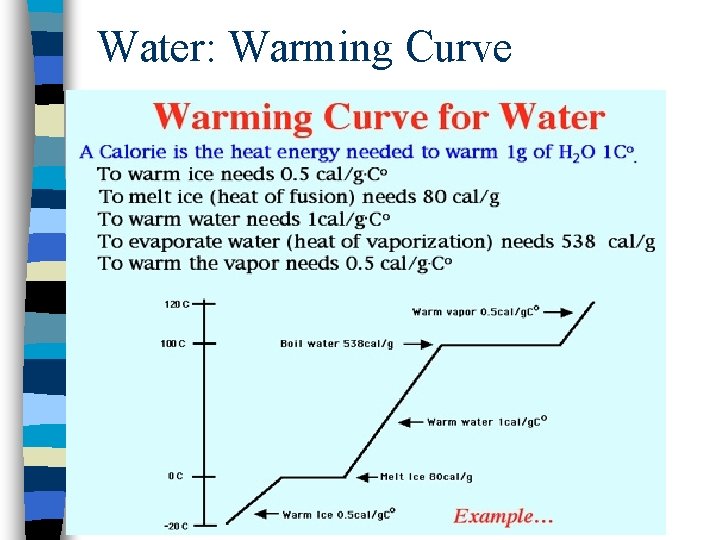
Water: Warming Curve

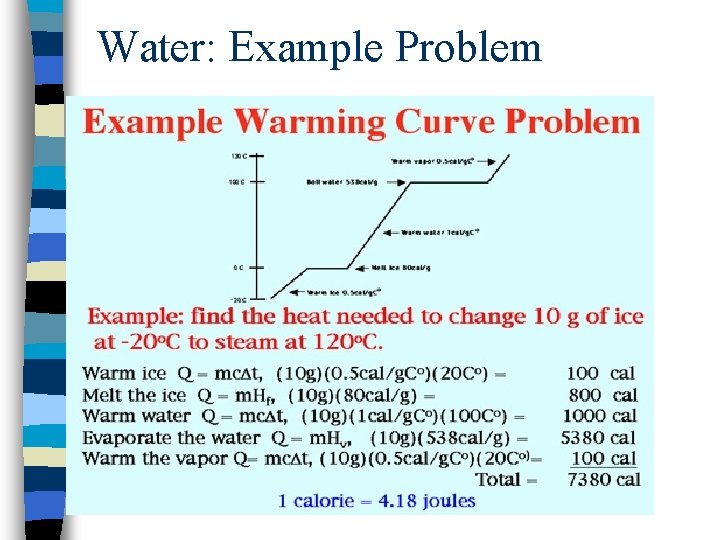
Water: Example Problem

Latent Heat Objectives are to: n Describe what is meant by ‘latent heat‘ n Compare and contrast the 3 phases of matter n Relatent heat to phase changes

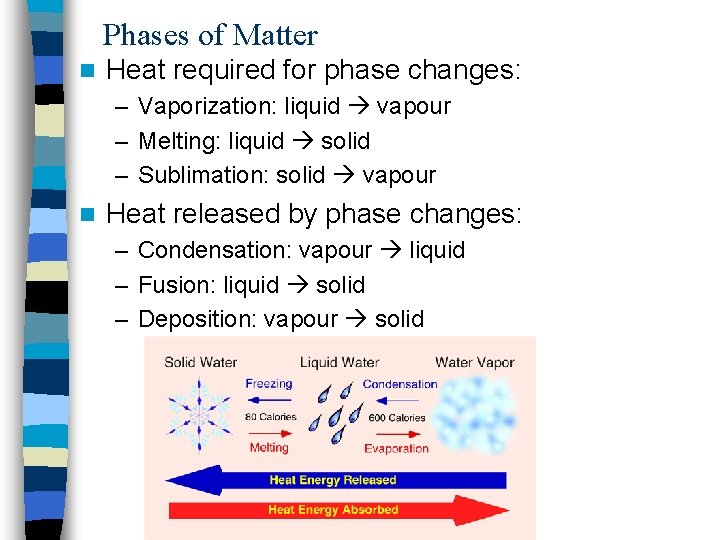
Phases of Matter n Heat required for phase changes: – Vaporization: liquid vapour – Melting: liquid solid – Sublimation: solid vapour n Heat released by phase changes: – Condensation: vapour liquid – Fusion: liquid solid – Deposition: vapour solid

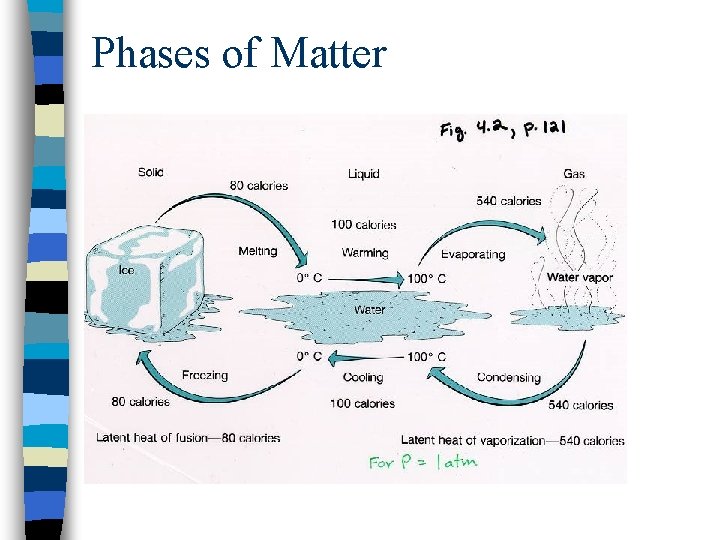
Phases of Matter

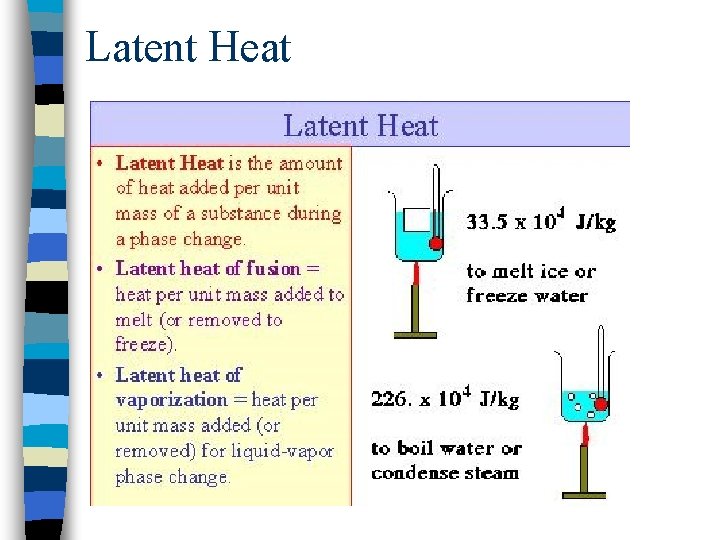
Latent Heat

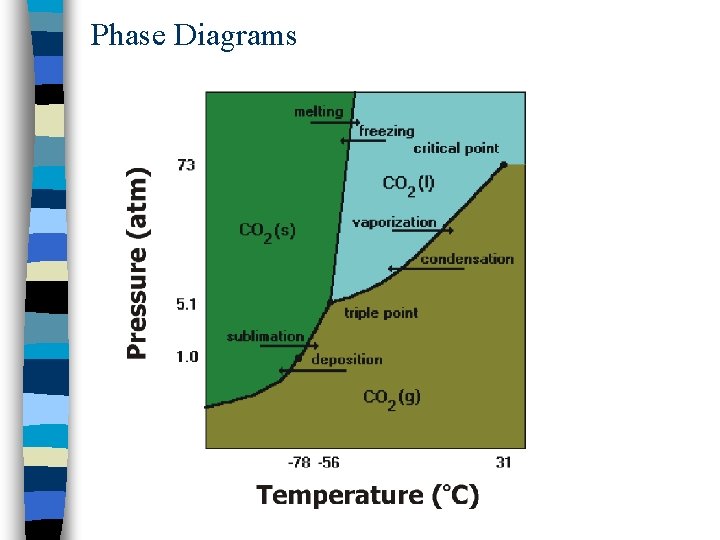
Phase Diagrams

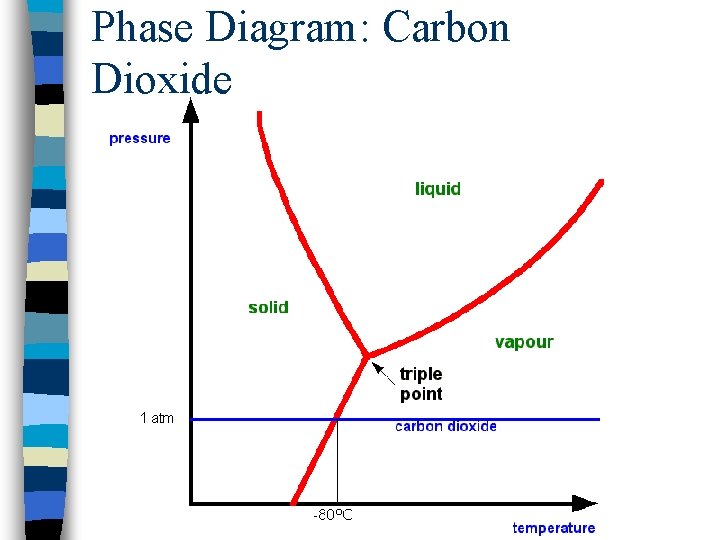
Phase Diagrams n Visual representation of phase changes n Triple point: point at which all three phases coexist n Curves branching out from this point separate phase regions: – Fusion curve: solid-liquid boundary – Vaporization curve: liquid-gas boundary – Sublimation curve: solid-gas boundary

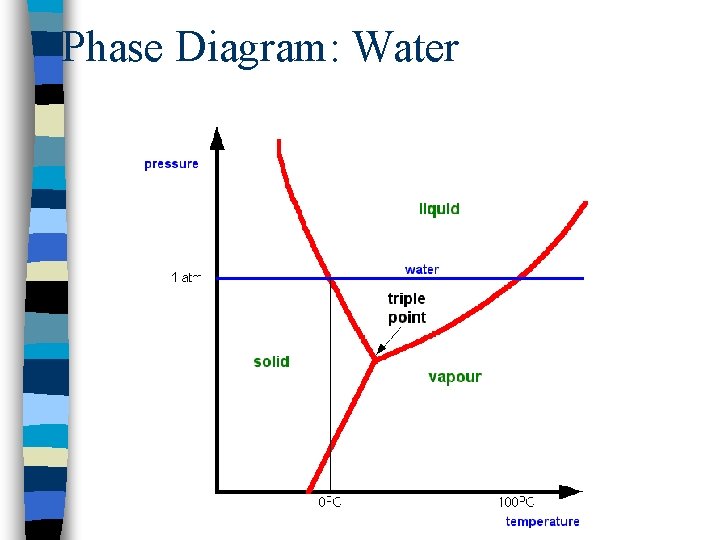
Phase Diagram: Water

Phase Diagram: Carbon Dioxide

Methods of Heat Transfer Objectives are to: n describe three methods of heat transfer n Give practical/environmental examples of each

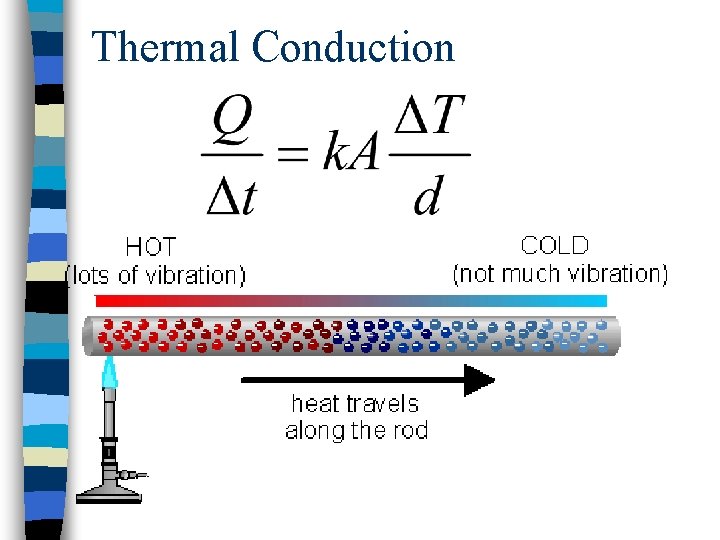
Thermal Conduction

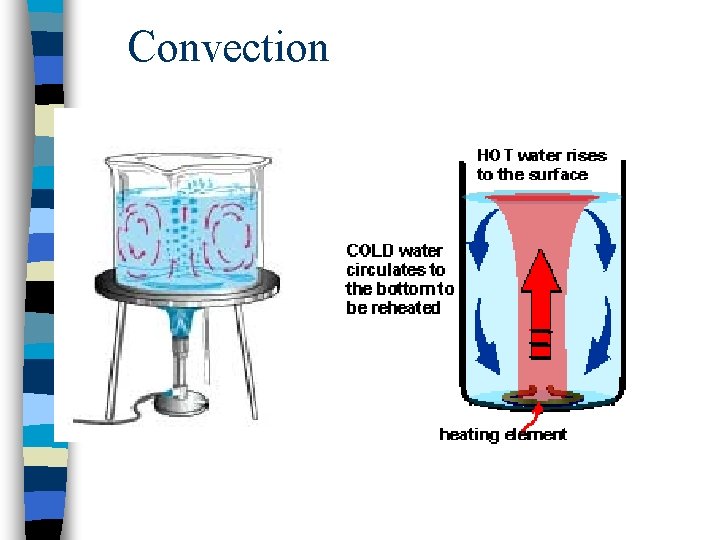
Convection

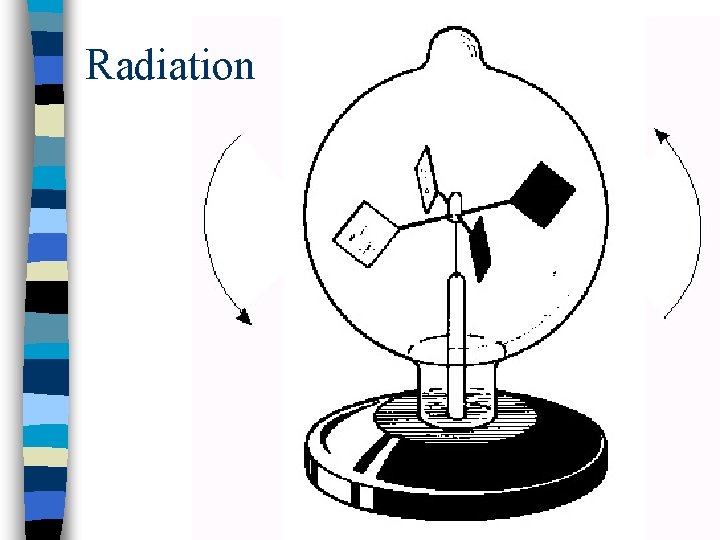
Radiation n Heat transfer by electromagnetic waves n Does not need a material medium n Black body: perfect absorber perfect emitter (at all wavelengths)

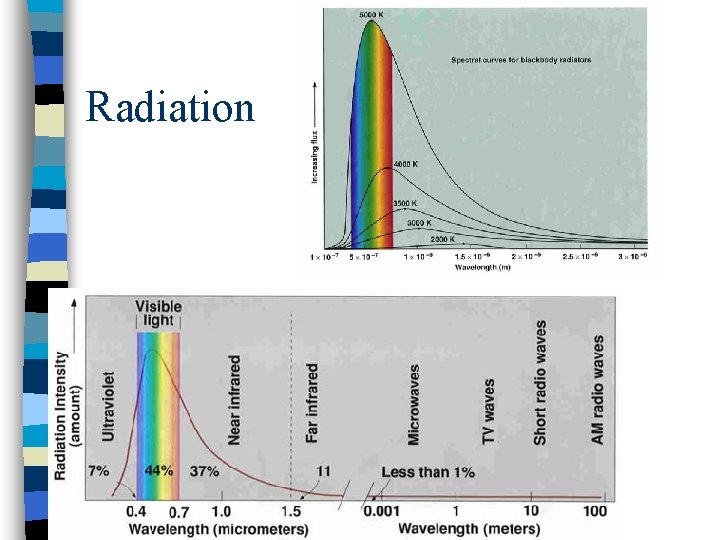
Radiation

Convection

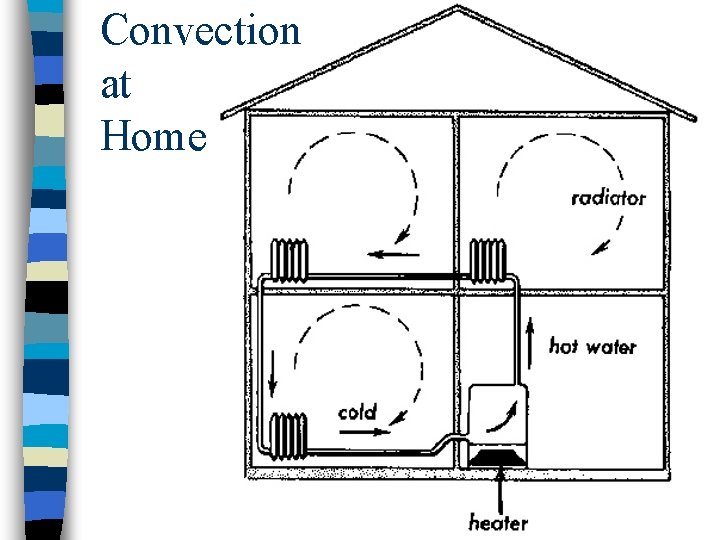
Convection at Home

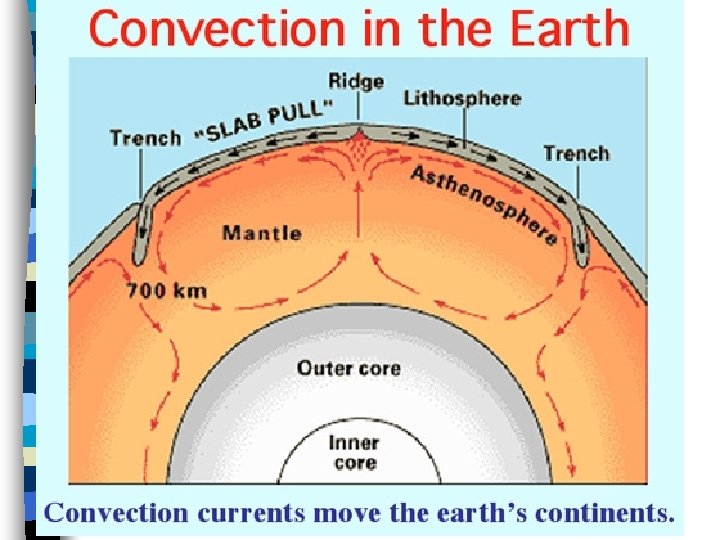
Convection

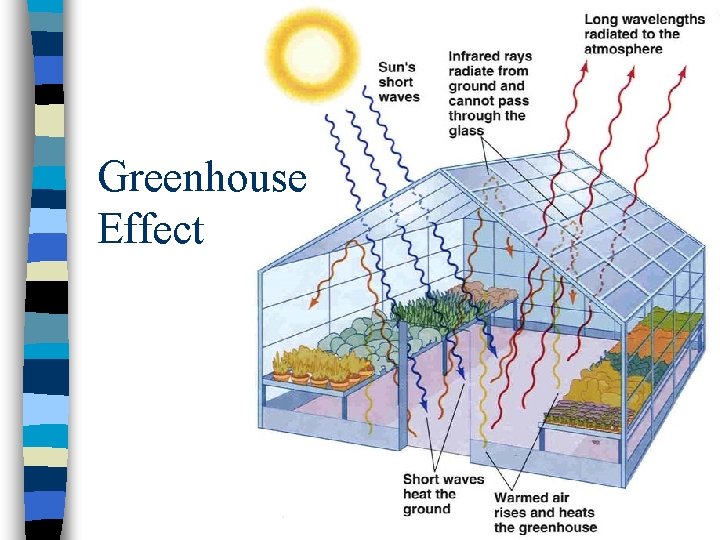
Greenhouse Effect

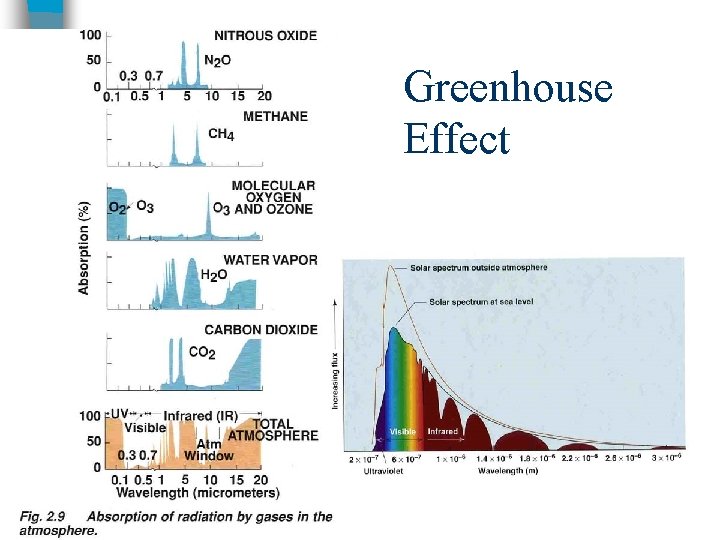
Greenhouse Effect

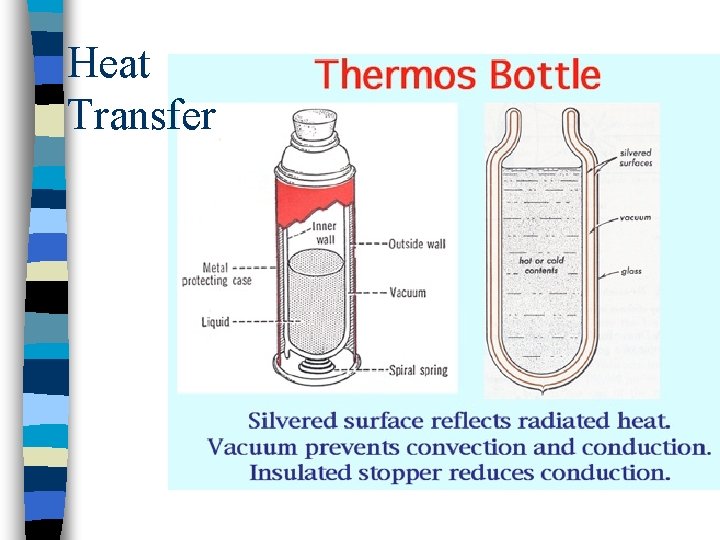
Heat Transfer

Radiation