Heat CHAPTER 10 1 TEMPERATURE AND THERMAL EQUILIBRIUM
Heat CHAPTER 10 -1 TEMPERATURE AND THERMAL EQUILIBRIUM
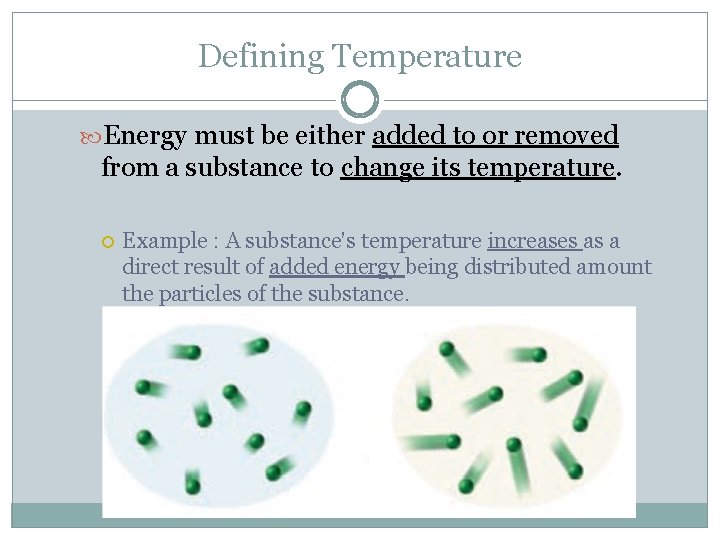
Defining Temperature Energy must be either added to or removed from a substance to change its temperature. Example : A substance’s temperature increases as a direct result of added energy being distributed amount the particles of the substance.
Thermal Equilibrium occurs when two objects in physical contact have the same temperatures. Thermal Equilibrium is the basis for measuring temperature with a thermometer.
Measuring Temperature units depend on the scale used. Scales we use are Fahrenheit (F), Celsius (C ), and Kelvin and (K). Formula to Convert from Celsius to Fahrenheit : Tf = (9/5) Tc + 32. 0 Fahrenheit temperature = (9/5 x Celsius Temperature) +32. 0 Formula for Celsius to Kelvin : T = Tc + 273. 15 Kelvin Temperature = Celsius Temperature + 273. 15
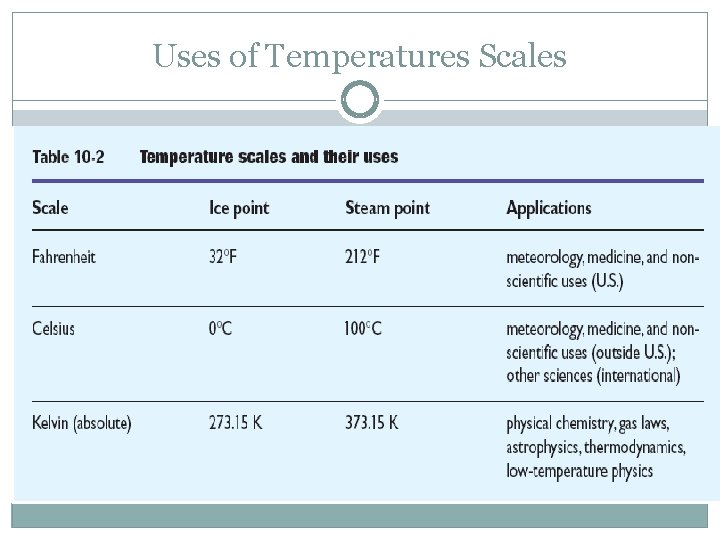
Uses of Temperatures Scales

Guided Practice Go to pg. 363 in Book Sample Problem 10 A
Heat CHAPTER 10 -2 DEFINING HEAT
Heat and Energy Heat is the energy transfer between substances. Symbol : Q Energy is transferred as heat from objects with higher temperatures (hot) to those with lower temperatures (cold). This transfer of energy changes an object’s temperature.
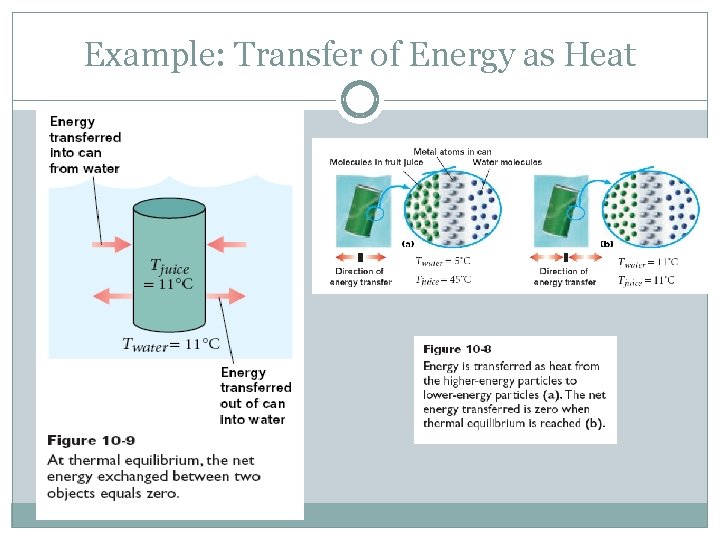
Example: Transfer of Energy as Heat
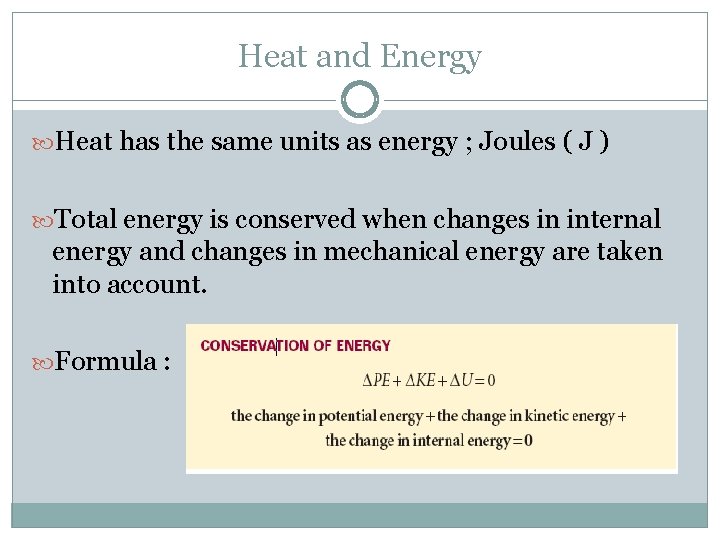
Heat and Energy Heat has the same units as energy ; Joules ( J ) Total energy is conserved when changes in internal energy and changes in mechanical energy are taken into account. Formula :

Guided Practice Open Books to Pg. 369 Sample 10 B

Chapter 10 SECTION 3 CHANGES IN TEMPERATURE AND PHASE
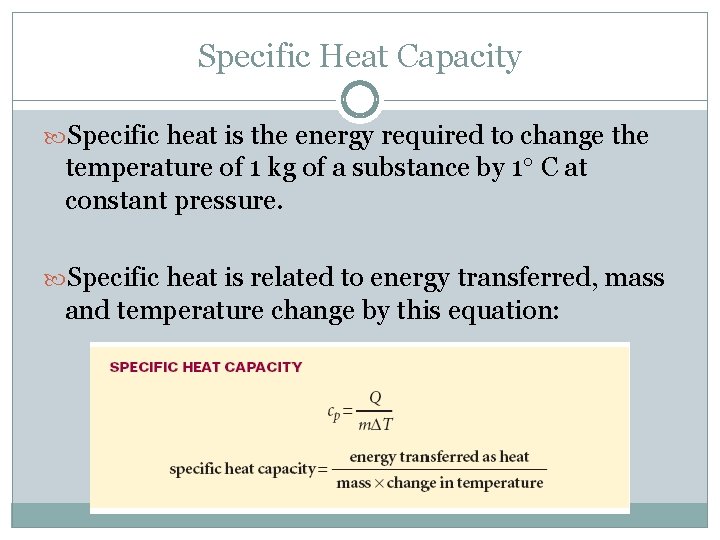
Specific Heat Capacity Specific heat is the energy required to change the temperature of 1 kg of a substance by 1 C at constant pressure. Specific heat is related to energy transferred, mass and temperature change by this equation:
Determining Specific Heat Capacity Calorimetry is used to determine specific heat.
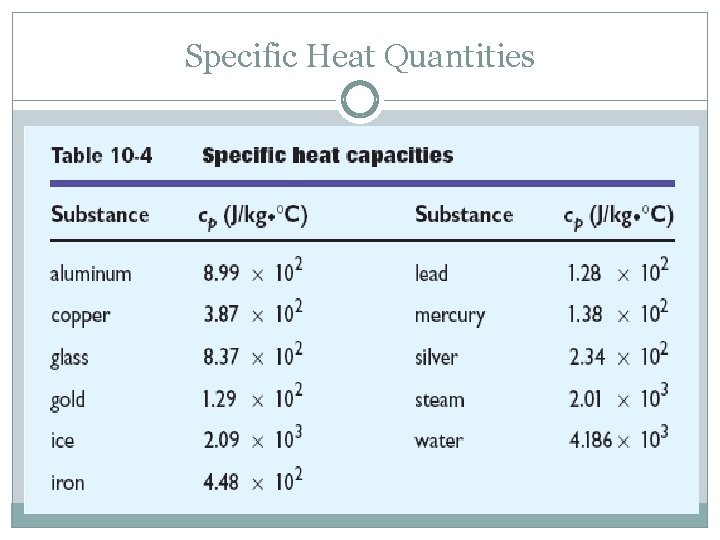
Specific Heat Quantities

Guided Practice Open Books to Pg. 373 Sample 10 C
Latent Heat When energy is used to melt a substance, that energy goes into rearranging the molecules (heat of fusion). When energy is used to vaporize a substance, that energy mostly goes into separating the molecules (heat of vaporization). Both heat of fusion and heat of vaporization are classified as Latent Heat. It takes more energy to vaporize than to melt a substance.
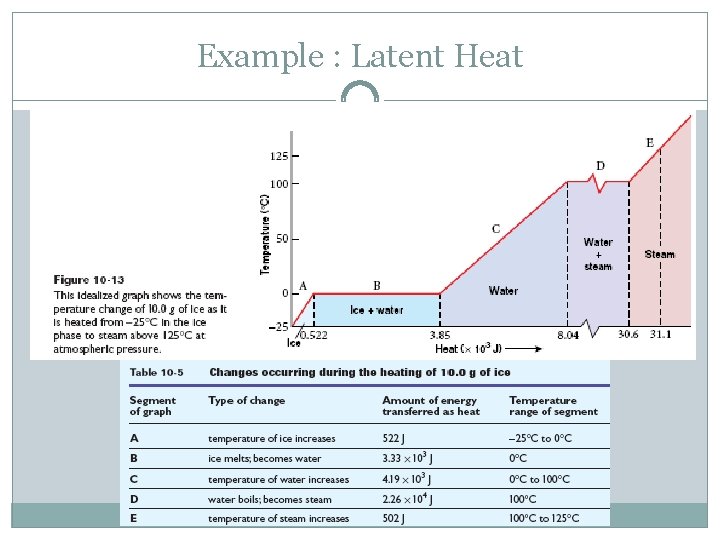
Example : Latent Heat
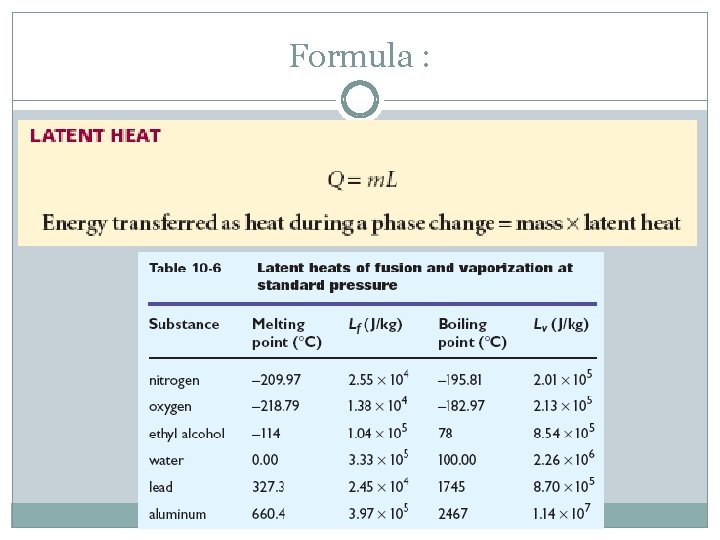
Formula :

Guided Practice: Go to page 380 in book Sample 10 D

Chapter 10 SECTION 4 CONTROLLING HEAT
Thermal Conduction Thermal conduction is the transfer of energy as heat through a material by particle collisions. Substances that rapidly transfer energy as heat are called thermal conductors. Examples : Metals – copper, silver, iron, steel Substances that slowly transfer energy as heat are called thermal insulators. Examples : asbestos, cork, ceramic, cardboard, fiberglass, wood , styrofoam, air , paper
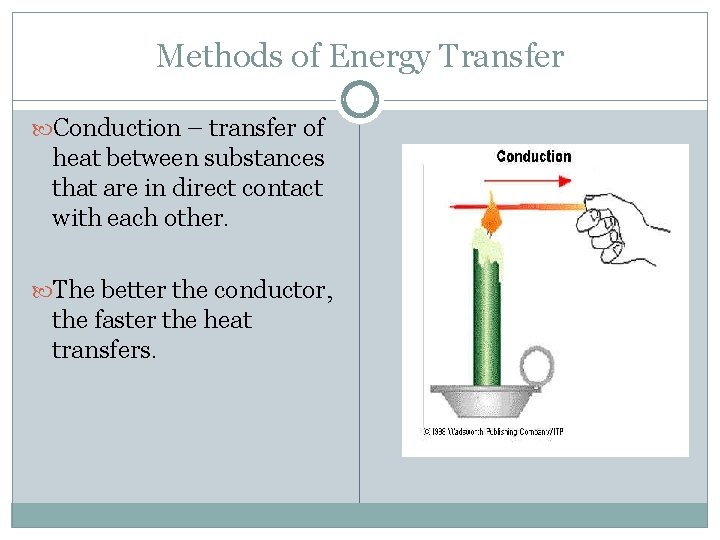
Methods of Energy Transfer Conduction – transfer of heat between substances that are in direct contact with each other. The better the conductor, the faster the heat transfers.
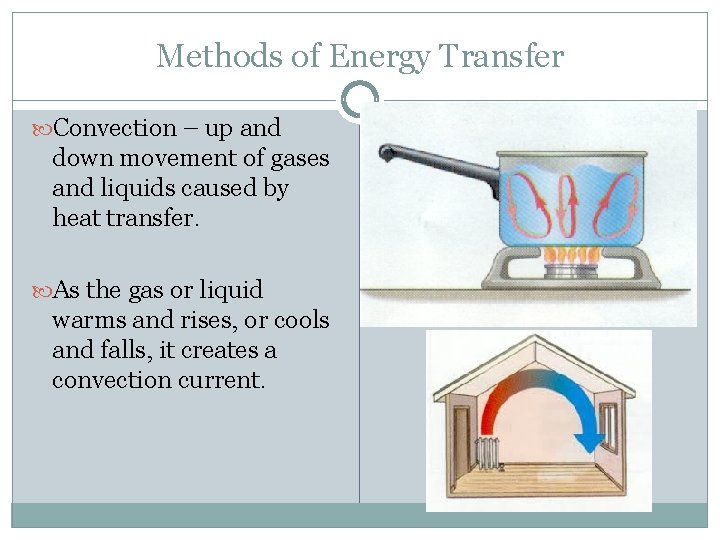
Methods of Energy Transfer Convection – up and down movement of gases and liquids caused by heat transfer. As the gas or liquid warms and rises, or cools and falls, it creates a convection current.
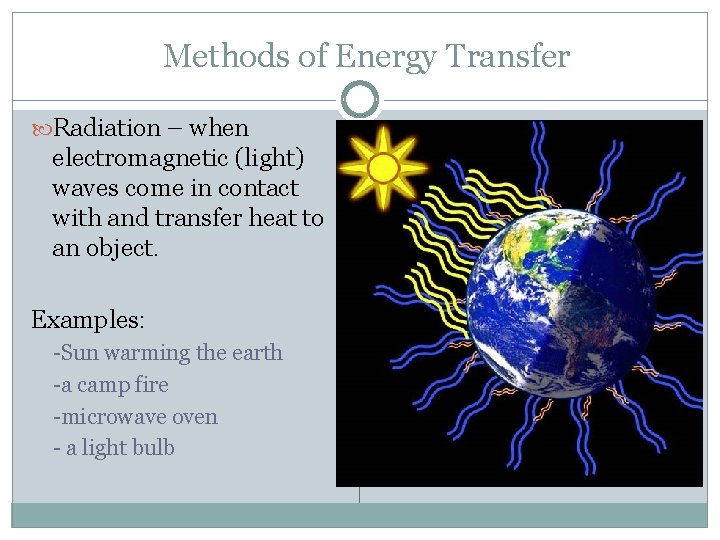
Methods of Energy Transfer Radiation – when electromagnetic (light) waves come in contact with and transfer heat to an object. Examples: -Sun warming the earth -a camp fire -microwave oven - a light bulb
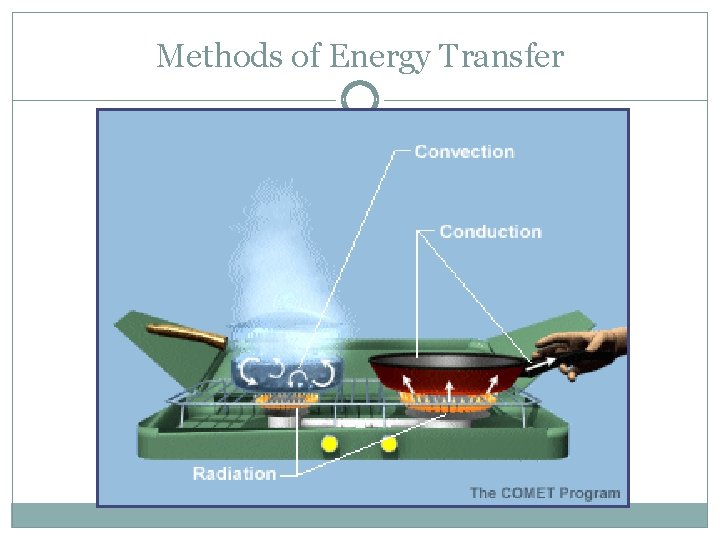
Methods of Energy Transfer
- Slides: 26