Heat Capacity of Solids and Electrical Conduction Homework




- Slides: 16

Heat Capacity of Solids and Electrical Conduction Homework: Chapter 11 Krane Problems: 13, 14, 15, 16, 17, 23, 28, 33, 34, 35 Due Monday December 1 st 1

Heat capacity revisited So far we have considered the heat capacity of the electron gas, but a solid is also made up of atoms Let’s recall the results for the electron gas… 2

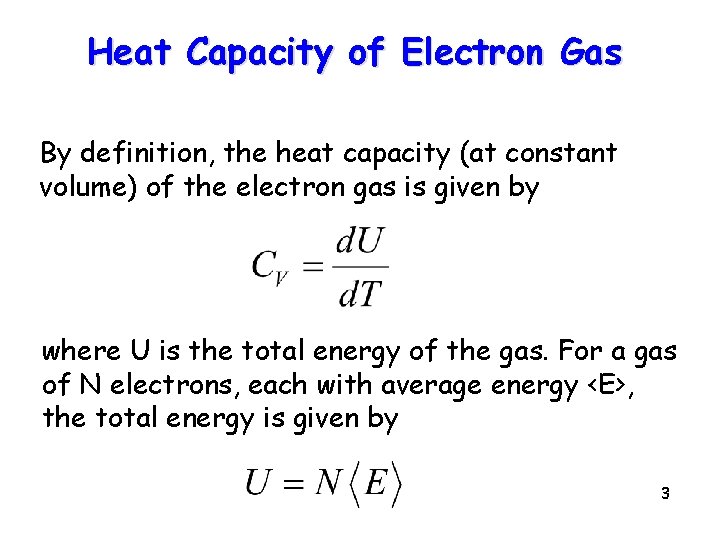
Heat Capacity of Electron Gas By definition, the heat capacity (at constant volume) of the electron gas is given by where U is the total energy of the gas. For a gas of N electrons, each with average energy <E>, the total energy is given by 3

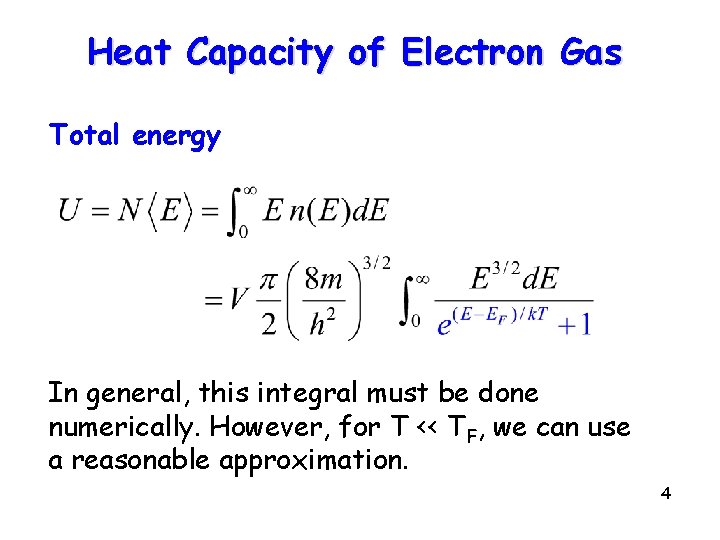
Heat Capacity of Electron Gas Total energy In general, this integral must be done numerically. However, for T << TF, we can use a reasonable approximation. 4

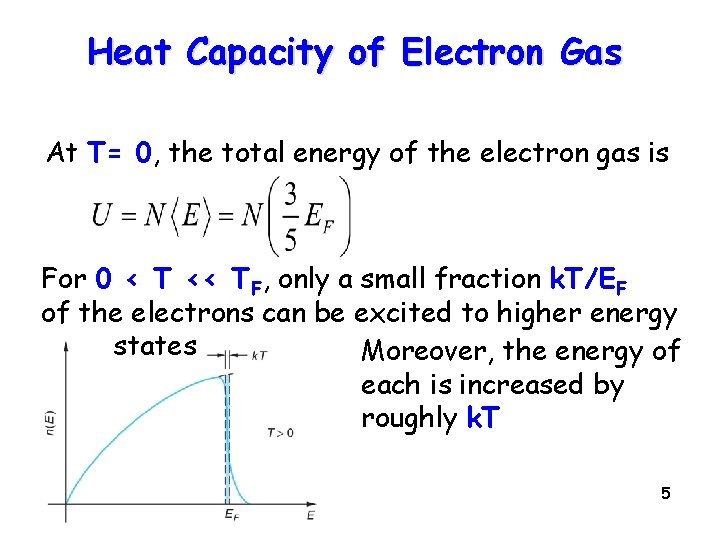
Heat Capacity of Electron Gas At T= 0, the total energy of the electron gas is For 0 < T << TF, only a small fraction k. T/EF of the electrons can be excited to higher energy states Moreover, the energy of each is increased by roughly k. T 5

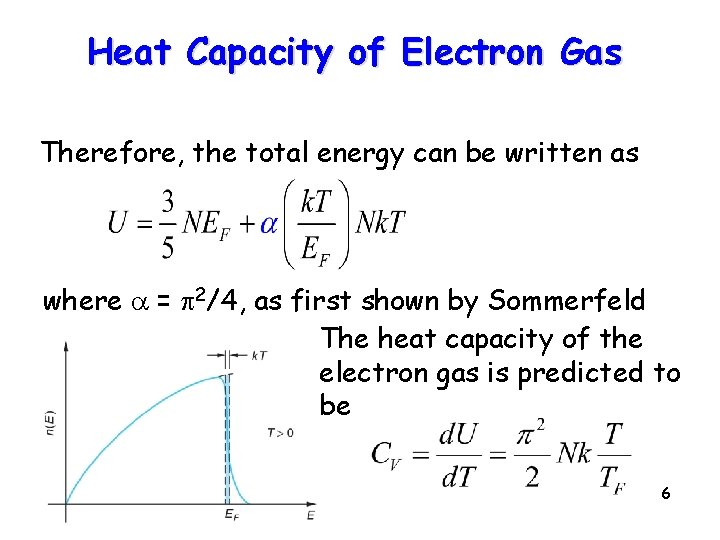
Heat Capacity of Electron Gas Therefore, the total energy can be written as where a = p 2/4, as first shown by Sommerfeld The heat capacity of the electron gas is predicted to be 6

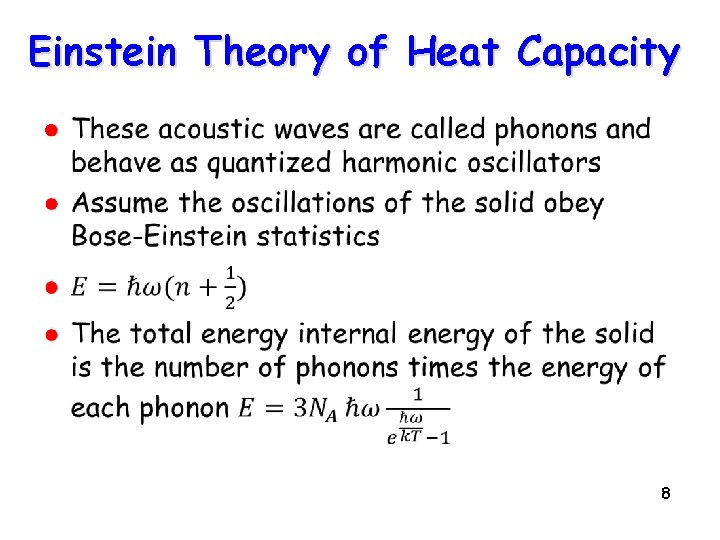
Contribution from lattice Electron gas contribution How about the lattice? l l l Lattice can undergo vibrations as temperature is increased The quantum of a lattice vibration is called a “phonon” The phonons actually dominate the heat capacity of solids at all but the lowest temps 7

Einstein Theory of Heat Capacity l 8

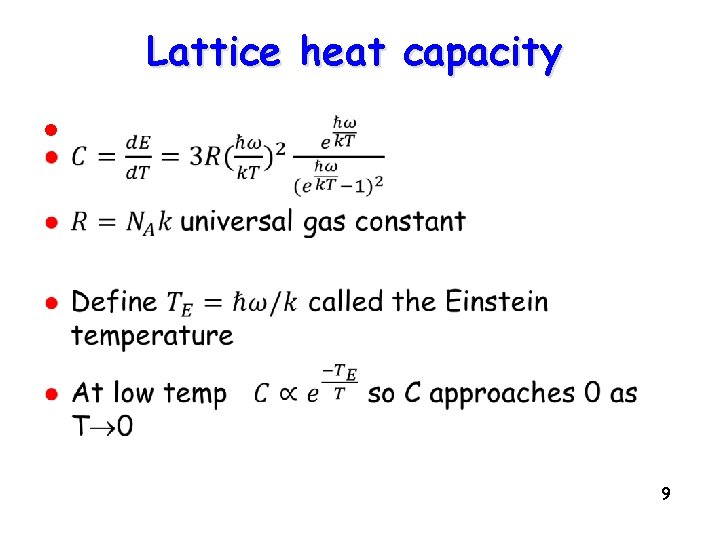
Lattice heat capacity l 9

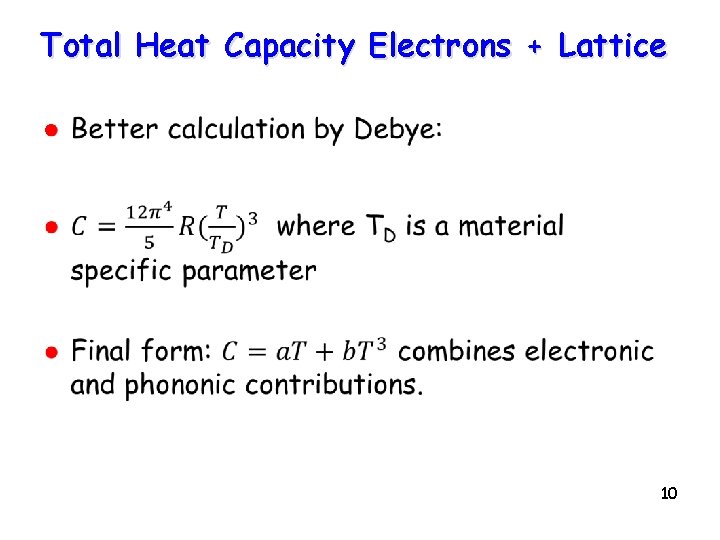
Total Heat Capacity Electrons + Lattice l 10

Quantum Theory of Conduction In a very large (strictly infinite) perfect crystal, calculations show that electrons suffer no scattering. That is, their mean free path is infinite. In real crystals, electrons scatter off imperfections and thermal vibrations of the lattice ions 11

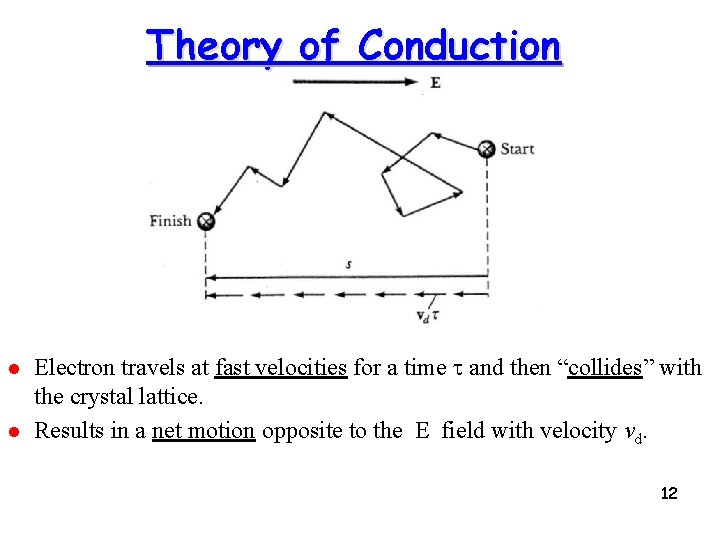
Theory of Conduction l l Electron travels at fast velocities for a time t and then “collides” with the crystal lattice. Results in a net motion opposite to the E field with velocity vd. 12

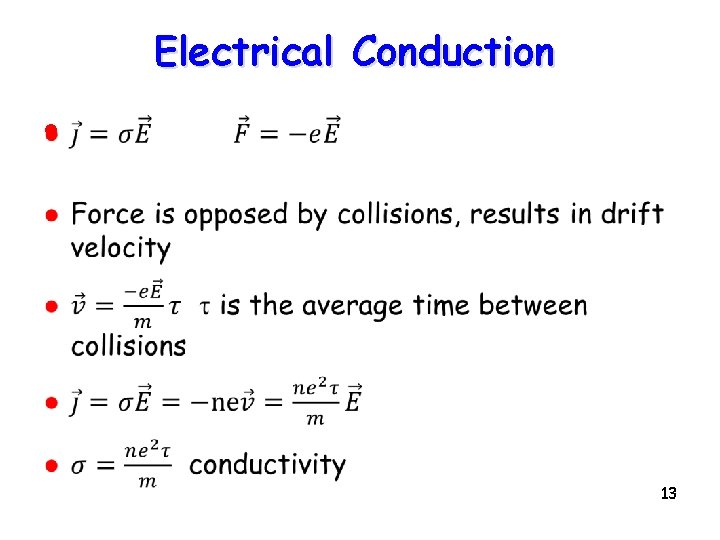
Electrical Conduction l 13

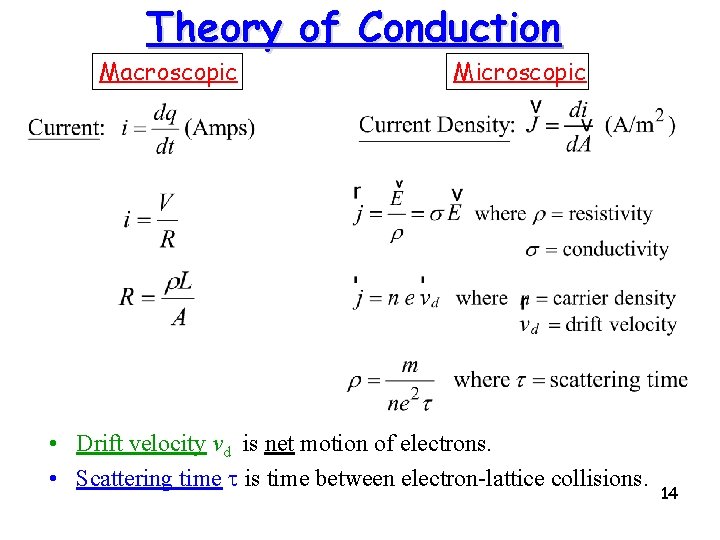
Theory of Conduction Macroscopic Microscopic • Drift velocity vd is net motion of electrons. • Scattering time t is time between electron-lattice collisions. 14

Resistivity l resistivity as a function of n and t Temperature dependence • Metal: Resistance increases with Temperature. Why? Temp t, n same (same # conduction electrons) r 15

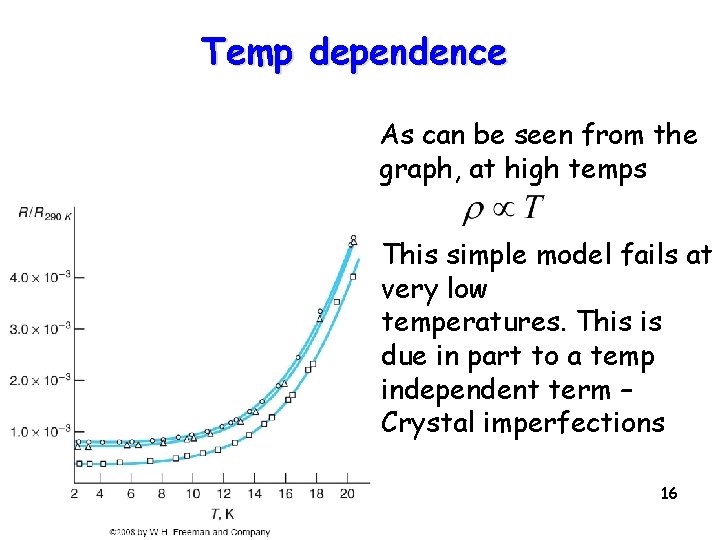
Temp dependence As can be seen from the graph, at high temps This simple model fails at very low temperatures. This is due in part to a temp independent term – Crystal imperfections 16
 Heat transfer objectives
Heat transfer objectives Electrical analogy in heat transfer
Electrical analogy in heat transfer Specific heat capacity
Specific heat capacity All people enjoy time magazine
All people enjoy time magazine Heat conduction experiments
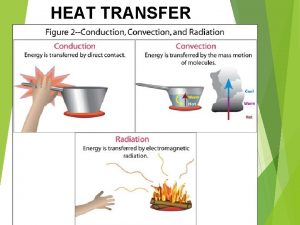
Heat conduction experiments What is heat transfer conduction convection and radiation
What is heat transfer conduction convection and radiation Example of heat transfer by radiation
Example of heat transfer by radiation Does heat travel in a straight line
Does heat travel in a straight line Radiation heat transfer
Radiation heat transfer Electrical conductivity of solids
Electrical conductivity of solids Production units have an optimal rate of output where:
Production units have an optimal rate of output where: Energy transfer
Energy transfer Transient heat transfer in industry
Transient heat transfer in industry Types of heat transfers
Types of heat transfers Evaporation heat loss newborn
Evaporation heat loss newborn Fourier's law heat transfer
Fourier's law heat transfer Heat energy is transferred by conduction whenever molecules
Heat energy is transferred by conduction whenever molecules