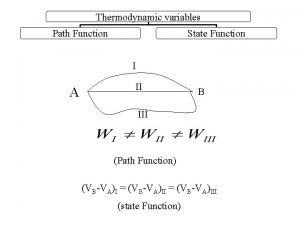
Heat Capacity Heat In Heat into a system
![Heat In Heat into a system ] Temperature change is due to heat (Q). Heat In Heat into a system ] Temperature change is due to heat (Q).](https://slidetodoc.com/presentation_image_h/f0db275f5e1d3e7c0297a4d09abb4f8a/image-2.jpg)
![Heat Capacity ] The ratio of the change in heat to the change in Heat Capacity ] The ratio of the change in heat to the change in](https://slidetodoc.com/presentation_image_h/f0db275f5e1d3e7c0297a4d09abb4f8a/image-3.jpg)
![Specific Heat ] The ratio of the change in heat to the change in Specific Heat ] The ratio of the change in heat to the change in](https://slidetodoc.com/presentation_image_h/f0db275f5e1d3e7c0297a4d09abb4f8a/image-4.jpg)
![Take a Shower ] ] You are last to use the shower today and Take a Shower ] ] You are last to use the shower today and](https://slidetodoc.com/presentation_image_h/f0db275f5e1d3e7c0297a4d09abb4f8a/image-5.jpg)
![Equilibrium Temperature ] Two systems in thermal contact will adjust to reach the same Equilibrium Temperature ] Two systems in thermal contact will adjust to reach the same](https://slidetodoc.com/presentation_image_h/f0db275f5e1d3e7c0297a4d09abb4f8a/image-6.jpg)
![Doing Dishes ] A 1. 5 kg aluminum pan is heated to 180 C Doing Dishes ] A 1. 5 kg aluminum pan is heated to 180 C](https://slidetodoc.com/presentation_image_h/f0db275f5e1d3e7c0297a4d09abb4f8a/image-7.jpg)
![Heat of Transformation heat of fusion heat of vaporization ] As a system reaches Heat of Transformation heat of fusion heat of vaporization ] As a system reaches](https://slidetodoc.com/presentation_image_h/f0db275f5e1d3e7c0297a4d09abb4f8a/image-8.jpg)
![Latent Heat ] The heat of transformation per unit mass is called the latent Latent Heat ] The heat of transformation per unit mass is called the latent](https://slidetodoc.com/presentation_image_h/f0db275f5e1d3e7c0297a4d09abb4f8a/image-9.jpg)
![Summer Cooler ] ] 200 g of ice at -10 C is added to Summer Cooler ] ] 200 g of ice at -10 C is added to](https://slidetodoc.com/presentation_image_h/f0db275f5e1d3e7c0297a4d09abb4f8a/image-10.jpg)
- Slides: 10

Heat Capacity
![Heat In Heat into a system Temperature change is due to heat Q Heat In Heat into a system ] Temperature change is due to heat (Q).](https://slidetodoc.com/presentation_image_h/f0db275f5e1d3e7c0297a4d09abb4f8a/image-2.jpg)
Heat In Heat into a system ] Temperature change is due to heat (Q). • If an object’s temperature increases it gains heat. • If an object’s temperature decreases it loses heat. ] This is comparable to work done on a system.
![Heat Capacity The ratio of the change in heat to the change in Heat Capacity ] The ratio of the change in heat to the change in](https://slidetodoc.com/presentation_image_h/f0db275f5e1d3e7c0297a4d09abb4f8a/image-3.jpg)
Heat Capacity ] The ratio of the change in heat to the change in temperature is the heat capacity (C). • • • Depends on material Depends on the mass Measured in J/K
![Specific Heat The ratio of the change in heat to the change in Specific Heat ] The ratio of the change in heat to the change in](https://slidetodoc.com/presentation_image_h/f0db275f5e1d3e7c0297a4d09abb4f8a/image-4.jpg)
Specific Heat ] The ratio of the change in heat to the change in temperature is the heat capacity (C). • Depends on material • independent of the mass • Measured in J/kg-K Material Mercury Copper Steel Granite Aluminum Wood Ice Water Specific heat 140 J/kg-K 390 J/kg-K 500 J/kg-K 840 J/kg-K 900 J/kg-K 1400 J/kg-K 2100 J/kg-K 4200 J/kg-K
![Take a Shower You are last to use the shower today and Take a Shower ] ] You are last to use the shower today and](https://slidetodoc.com/presentation_image_h/f0db275f5e1d3e7c0297a4d09abb4f8a/image-5.jpg)
Take a Shower ] ] You are last to use the shower today and the temperature in the water heater has dropped to 18 C from the normal temperature of 50 C. The heater holds 150 kg and has a 50 k. W heating coil. How long should you wait before starting your shower? Ø Find the heat required. Ø DQ = mc. DT = (150 kg) (4200 J/kg-K) ( 32 C) Ø DQ = 2. 1 x 107 J Ø Find the time from the power, and set the heat to the work. Ø Ø P = DW/Dt t = DQ / P (2. 1 x 107 J) / (5 x 104 W) t = 4000 s = 1. 1 hr
![Equilibrium Temperature Two systems in thermal contact will adjust to reach the same Equilibrium Temperature ] Two systems in thermal contact will adjust to reach the same](https://slidetodoc.com/presentation_image_h/f0db275f5e1d3e7c0297a4d09abb4f8a/image-6.jpg)
Equilibrium Temperature ] Two systems in thermal contact will adjust to reach the same temperature - thermal equilibrium ] If they are thermally insulated, all the heat goes from the hot system to the cold system. 1 2
![Doing Dishes A 1 5 kg aluminum pan is heated to 180 C Doing Dishes ] A 1. 5 kg aluminum pan is heated to 180 C](https://slidetodoc.com/presentation_image_h/f0db275f5e1d3e7c0297a4d09abb4f8a/image-7.jpg)
Doing Dishes ] A 1. 5 kg aluminum pan is heated to 180 C then placed into a sink with 8 kg water at 20 C. ] If no water boils, what is the equilibrium temperature? Ø Setup the equation for an unknown equilibrium temperature T. Ø Solve for T. Ø T = 26 C
![Heat of Transformation heat of fusion heat of vaporization As a system reaches Heat of Transformation heat of fusion heat of vaporization ] As a system reaches](https://slidetodoc.com/presentation_image_h/f0db275f5e1d3e7c0297a4d09abb4f8a/image-8.jpg)
Heat of Transformation heat of fusion heat of vaporization ] As a system reaches the point of a phase change heat no longer changes the temperature. ] The heat is used to change the phase.
![Latent Heat The heat of transformation per unit mass is called the latent Latent Heat ] The heat of transformation per unit mass is called the latent](https://slidetodoc.com/presentation_image_h/f0db275f5e1d3e7c0297a4d09abb4f8a/image-9.jpg)
Latent Heat ] The heat of transformation per unit mass is called the latent heat (L). • Measured in J/kg ] It takes as much energy to melt 1 g of ice as it does to raise the temperature from 0 C to 80 C. Material (f or v) Mercury (f) Lead (f) Uranium (f) Copper (f) Water (f) Latent Heat 11. 3 k. J/kg 24. 7 k. J/kg 82. 8 k. J/kg 205 k. J/kg 334 k. J/kg Oxygen (v) Water (v) 213 k. J/kg 2300 k. J/kg
![Summer Cooler 200 g of ice at 10 C is added to Summer Cooler ] ] 200 g of ice at -10 C is added to](https://slidetodoc.com/presentation_image_h/f0db275f5e1d3e7c0297a4d09abb4f8a/image-10.jpg)
Summer Cooler ] ] 200 g of ice at -10 C is added to 1. 0 kg of water at 15 C. ] Find the amount of heat needed to bring the ice to 0 C and melt it. • Qi = mc. DT + m. Lf • Qi = 70. 9 k. J Is there enough ice to cool the water to 0 C? ] Find the heat needed to cool the water down to 0 C. • Qw = mc. DT • Qw = 62. 8 k. J ] Yes, there’s enough ice.