HEAT CALCULATIONS AND DIAGRAMS Key Terms Heat Capacity









- Slides: 14

HEAT: CALCULATIONS AND DIAGRAMS

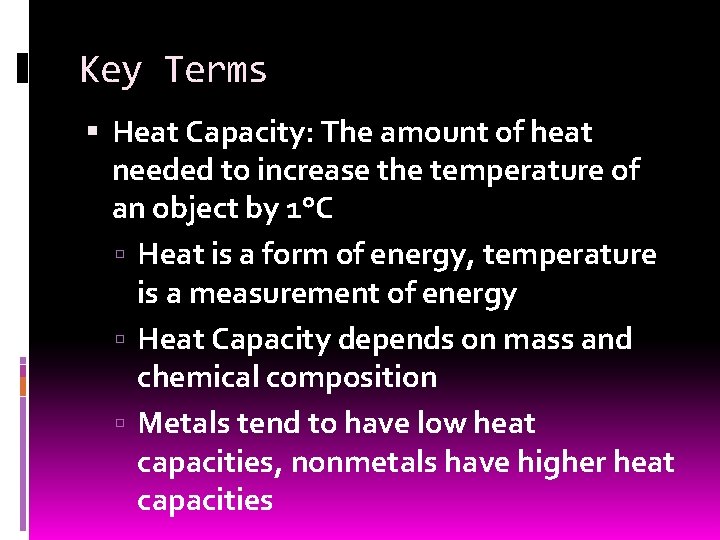
Key Terms Heat Capacity: The amount of heat needed to increase the temperature of an object by 1°C Heat is a form of energy, temperature is a measurement of energy Heat Capacity depends on mass and chemical composition Metals tend to have low heat capacities, nonmetals have higher heat capacities

How is Specific Heat Related? Specific Heat: amount of heat needed to raise 1 g by 1°C Measured in J/(g·°C) or cal/(g·°C) 1 Calorie = 1 kilocalorie = 1000 calories 1 Joule (J) = 0. 2930 cal 4. 184 J = 1 cal

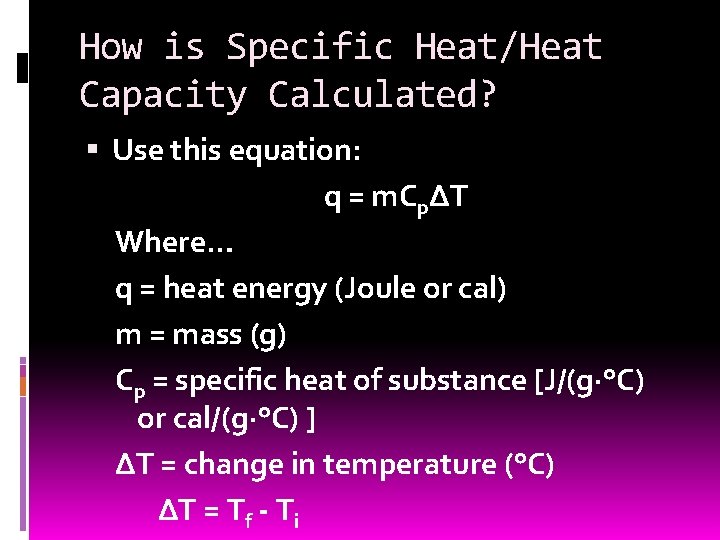
How is Specific Heat/Heat Capacity Calculated? Use this equation: q = m. CpΔT Where… q = heat energy (Joule or cal) m = mass (g) Cp = specific heat of substance [J/(g·°C) or cal/(g·°C) ] ΔT = change in temperature (°C) ΔT = Tf - Ti

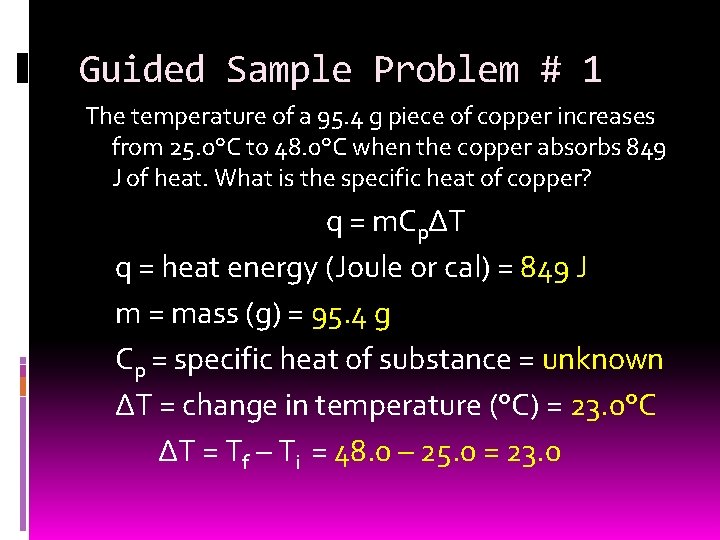
Guided Sample Problem # 1 The temperature of a 95. 4 g piece of copper increases from 25. 0°C to 48. 0°C when the copper absorbs 849 J of heat. What is the specific heat of copper? q = m. CpΔT q = heat energy (Joule or cal) = 849 J m = mass (g) = 95. 4 g Cp = specific heat of substance = unknown ΔT = change in temperature (°C) = 23. 0°C ΔT = Tf – Ti = 48. 0 – 25. 0 = 23. 0

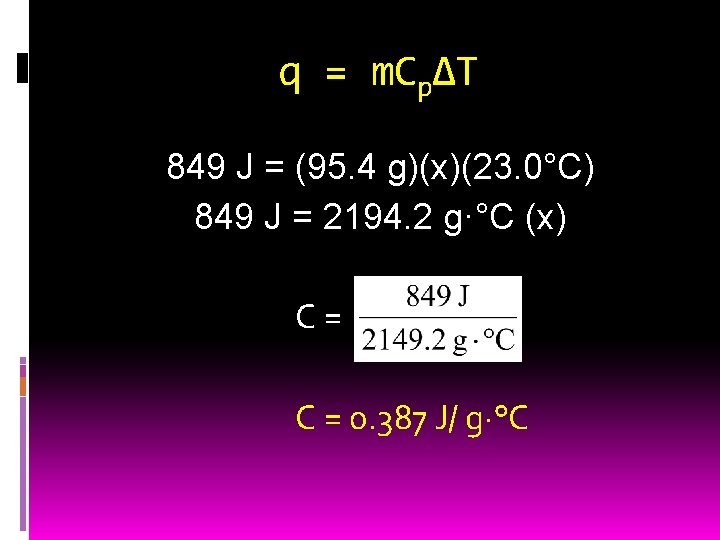
q = m. CpΔT 849 J = (95. 4 g)(x)(23. 0°C) 849 J = 2194. 2 g·°C (x) C= C = 0. 387 J/ g·°C

Guided Practice # 2 When 435 J of heat is added to 3. 4 g of olive oil at 21°C, the temperature increases to 85°C. What is the specific heat of olive oil?

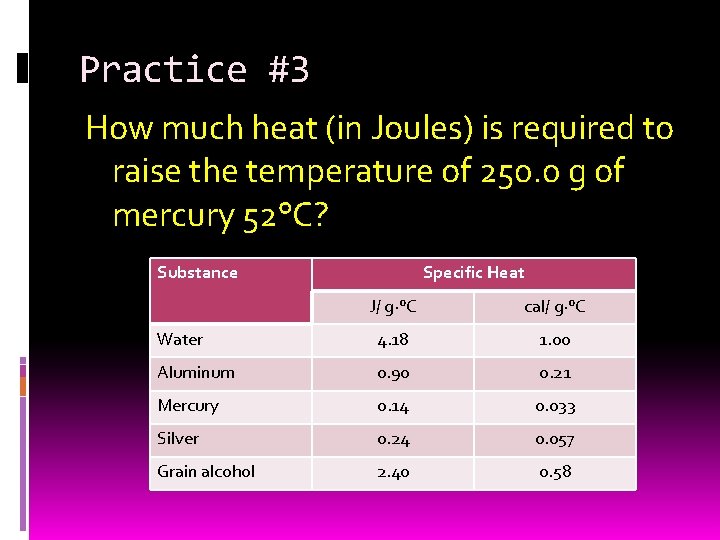
Practice #3 How much heat (in Joules) is required to raise the temperature of 250. 0 g of mercury 52°C? Substance Specific Heat J/ g·°C cal/ g·°C Water 4. 18 1. 00 Aluminum 0. 90 0. 21 Mercury 0. 14 0. 033 Silver 0. 24 0. 057 Grain alcohol 2. 40 0. 58

Heat of Fusion and Heat of Vaporization Heat of Fusion = amount of heat needed to melt/freeze a substance Uses the equation: q = m. Hf Heat of Vaporization = amount of heat needed to boil/condense a substance Uses the equation: q = m. Hv Both use the unit: J/g

Guided Practice #4 What is the amount of heat needed to melt 64 g of ice at 0 degrees Celsius?

Guided Practice #5 What is the heat of vaporization of 70 g of chlorine gas if 5, 600 J of heat is needed to boil a sample of the gas?

Converting Temperatures Kelvin scale = most precise temperature scale. Based on Absolute Zero (theoretical temperature) Relates to Celsius with the equation: K = C + 273

Guided Practice #6 What is the temperature in Kelvin of a sample with 120 degrees celsius?

How in the world do I decide which equation to use? If the problem talks about changing temperatures (or gives you 2 different temperatures), and uses the word heat: Use q = m. CpΔT If the question uses the words melt/freeze, boil/condense (or variations of those words) AND does not give you more than 1 temperature: Use q = m. Hf or q = m. Hv