Heat and Temperature What is heat The transfer




















- Slides: 29

Heat and Temperature

What is heat? • The transfer of thermal energy from one object to another because of a difference in temperature. • Heat flows from an area of high heat to an area low in heat. • Result of work that is not 100% efficient

What is temperature? • The measure of how hot or cold something is compared to a reference point • Average kinetic energy of particles in an object

What is thermal energy? • Total potential and kinetic energy in an object • Depends on mass, temperature, and phase of an object

What has more heat (or thermal energy)? A. An iceberg or B. A burning match

The Iceberg. Why? Because an iceberg is much bigger and has more total molecular vibrations than the small match. Heat involves temperature AND mass!!!

First Law of Thermodynamics Energy cannot be created or destroyed, but it can be transferred from one location to another and/or converted to another form of energy.

So, how is heat transferred?

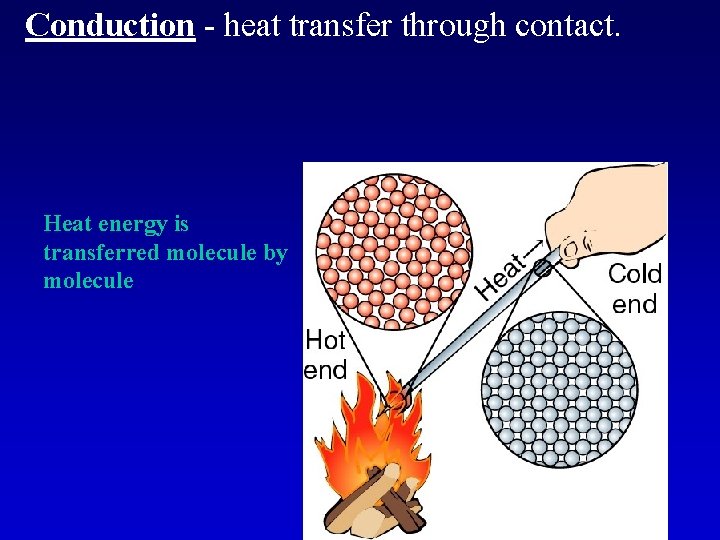
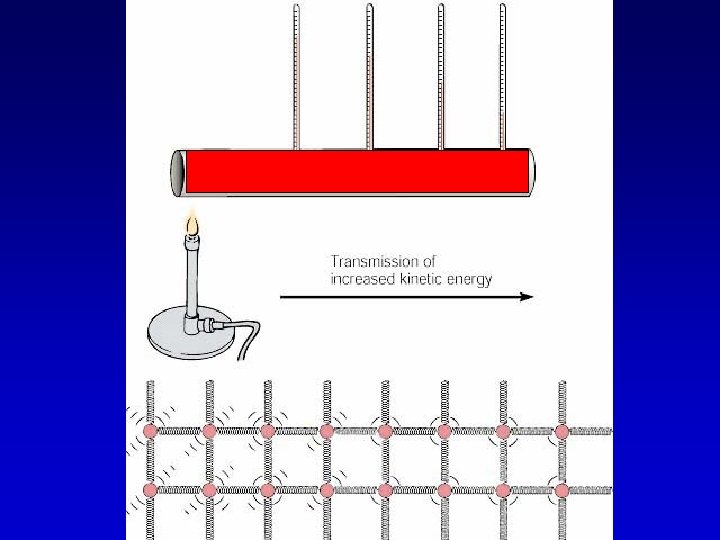
Conduction

Conduction - heat transfer through contact. Heat energy is transferred molecule by molecule


Some materials are good conductors while others are good insulators. – Conductors transfer energy very efficiently. – Insulators transfer energy very inefficiently.

This is fiberglass insulation. It keeps hot air out of your house during the summer. It keeps warm air inside your house during the winter.

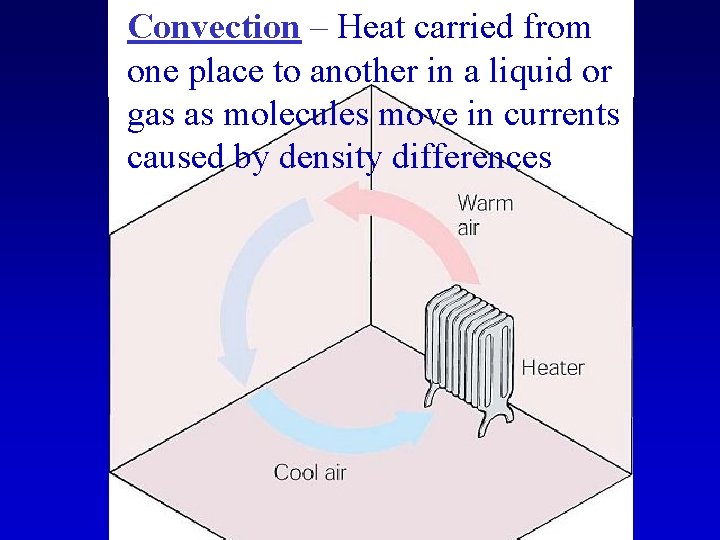
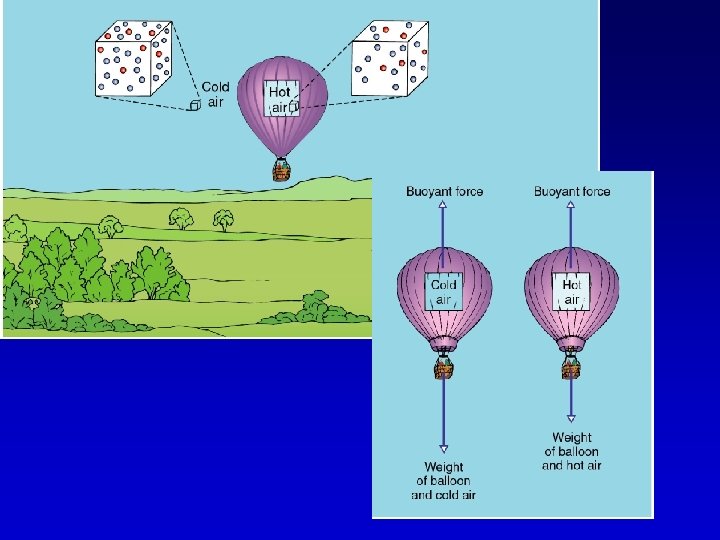
Convection

Convection – Heat carried from one place to another in a liquid or gas as molecules move in currents caused by density differences


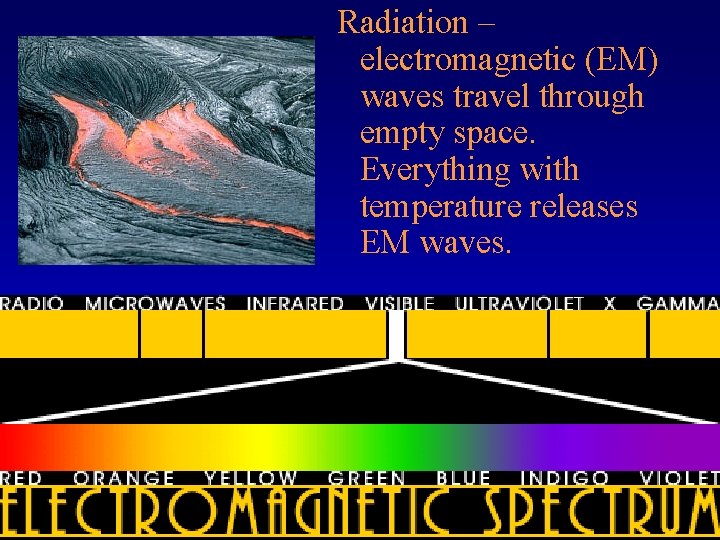
Radiation

Radiation – electromagnetic (EM) waves travel through empty space. Everything with temperature releases EM waves.

Three methods of heat transfer Conduction: – Transfer from one substance to another by direct contact of molecules. Example: When you touch a hot stove. • Convection: – Heat carried from one place to another in a liquid or gas as molecules move in currents caused by density differences. Example: Warm air rising. • Radiation: – Heat carried through empty space in the form of infrared rays. Example: When you face the sun and feel warmth on your face.

Second Law of Thermodynamics The entropy of a system always increases over time. Entropy: the measure of disorder Basically, heat can NEVER transfer from a cold object to a hot object

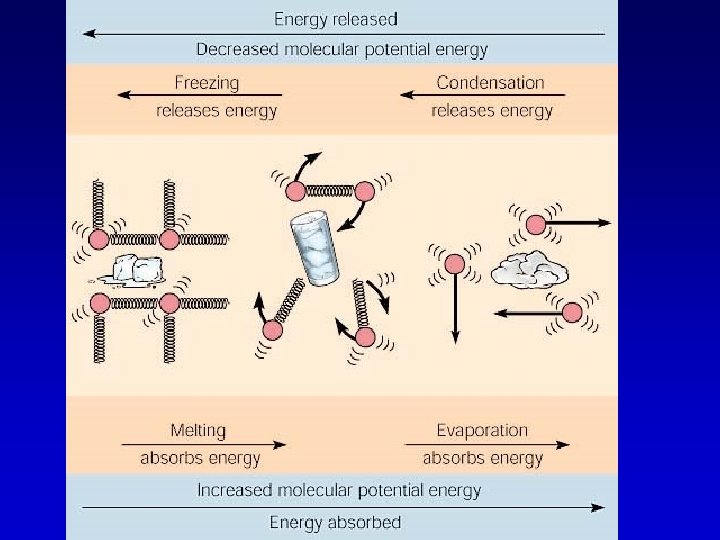
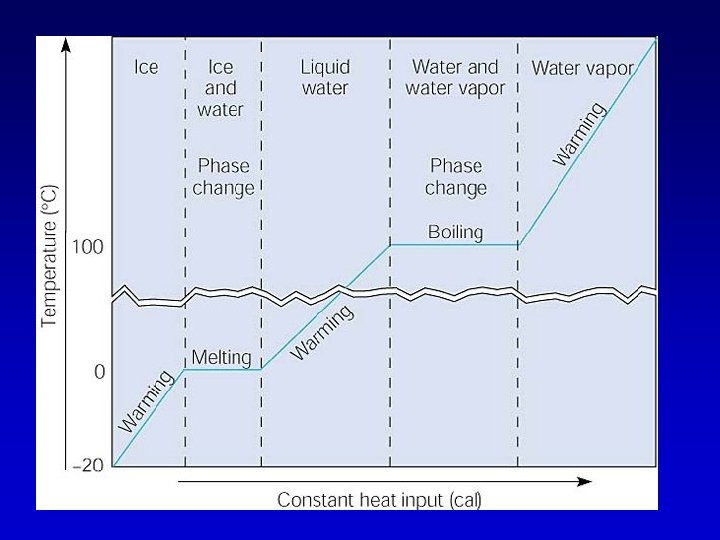
Heat energy and phase changes



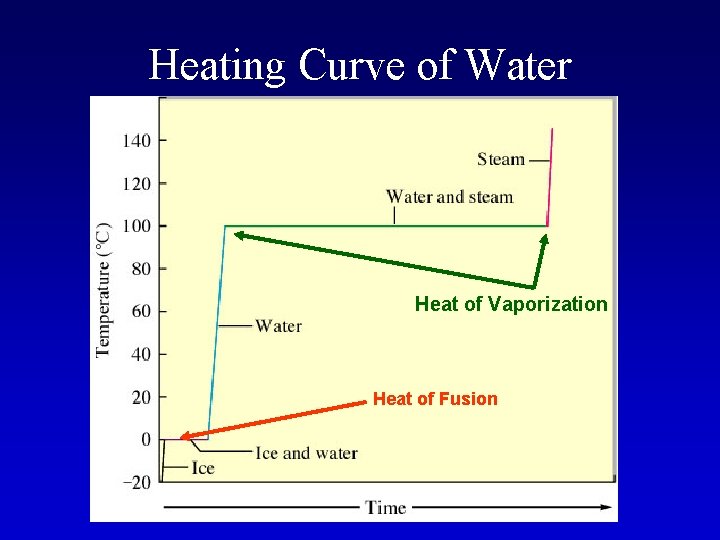
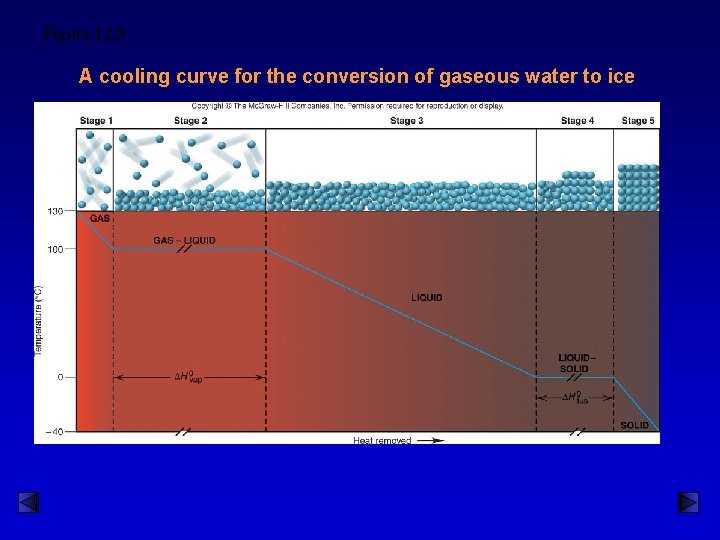
Heating Curve of Water Heat of Vaporization Heat of Fusion

Figure 12. 3 A cooling curve for the conversion of gaseous water to ice

Specific Heat • The ability of a substance to absorb heat. • The specific heat of a substance is the number of calories (or Joules) needed to raise the temperature of one gram of the substance one degree Celcius. • The units of Specific Heat are: – calories per gram degree Celsius – Joules per gram Kelvin

EQUATION • Heat gained or lost = (mass) (change in Temp) (specific heat) Q = m C ΔT Heat gained or lost Change in temp (final-initial) Mass (g) Specific Heat capacity

Specific Heat • Specific Heat of water: 1 calorie=4. 186 J/g °C Ex: Calculate the amount of heat needed to increase the temperature of 250 g of water from 20°C to 46°C.

Ex: Calculate the amount of heat needed to increase the temperature of 250 g of water from 20°C to 46°C. q = m x C x ΔT q = 250 g x 4. 18 J/go. C x 26 o. C q = 27, 209 J