HEAT AND TEMPERATURE CHAPTER 10 10 1 TEMPERATURE







- Slides: 14

HEAT AND TEMPERATURE CHAPTER 10

10. 1 TEMPERATURE

TEMPERATURE is ______ All the particles in matter are constantly moving…. . each particle has kinetic energy.

THERMOMETERS SOME ARE BASED ON THE PRINCIPLE THAT MATTER EXPANDS WHEN HEATED

THREE THERMOMETER SCALES

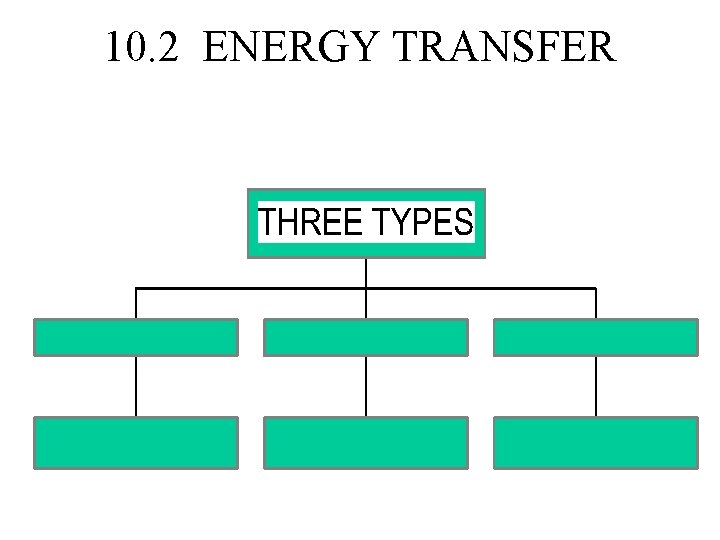
HEAT IS THE TRANSFER OF ENERGY BETWEEN OBJECTS OF DIFFERENT TEMPERATURE. • Heat always moves from a place of higher to one of lower temperature. • Objects with large mass will have more heat than ones with smaller mass.

SECTION 10. 1 REVIEW 1. Absolute zero is the temperature at which particles have almost no kinetic energy. 2. Particles in a cup of hot soup move faster because their temperature is higher. 3. A 100 K chamber is very cold. . -173 o. C so one would dress for cold…. the 100 o. C temperature means water is boiling…dress for extreme heat. 4. More heat transferred at 10 o. C to – 15 o. C because the temperature difference is greater. 5. a. Boiling water has higher temperature. b. Lake Michigan has more kinetic energy.

10. 2 ENERGY TRANSFER

SPECIFIC HEAT - ______ Use table 10 -1 to place the following substances in order of their specific heat with the one with the most first. • Copper, Lead, Water, Aluminum, Silver, Iron, Gold, Ethanol 1. 2. 3. 4. 5. 6. 7. 8.

Section 10. 2 - Review 1. a. Conduction – b. Convection – c. Radiation – 2. Gold, iron, water, air (use the table 10. 1) 3. Near the ceiling because heated air rises. 4. On the moon there is no air to heat up but there are shadows and where the sun does not shine it will be very cold.

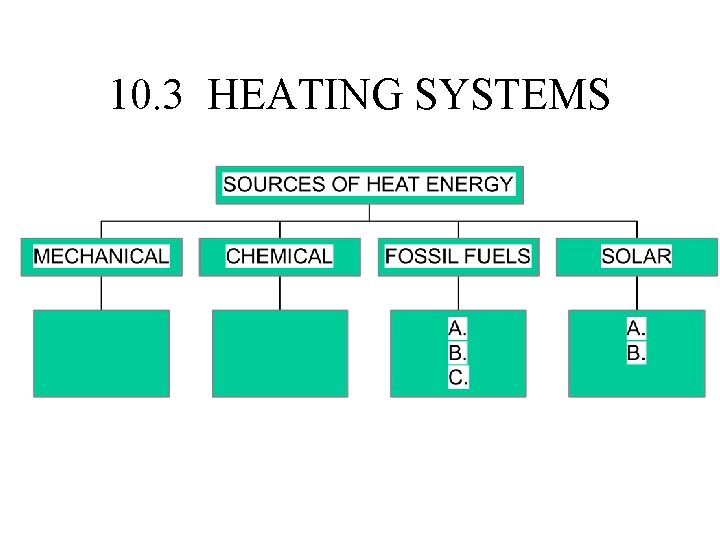
10. 3 HEATING SYSTEMS

• TOTAL ENERGY IS CONSERVED. • USABLE ENERGY DECREASES IN EVERY ENERGY TRANSFER. • INSULATION DECREASES UNDESIRABLE ENERGY TRANSFERS.

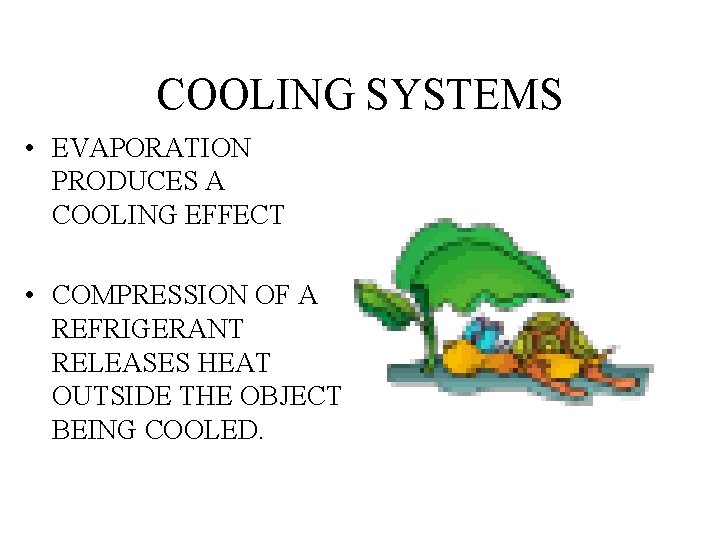
COOLING SYSTEMS • EVAPORATION PRODUCES A COOLING EFFECT • COMPRESSION OF A REFRIGERANT RELEASES HEAT OUTSIDE THE OBJECT BEING COOLED.

10. 3 REVIEW 1. Evaporation is a change of state from liquid to gas so this takes much energy. 2. HOT AIR/HOT WATER/STEAM/SOLAR 3. Some of the heat energy is used to heat surroundings. 4. Advantage would be lower energy bills…disadvantage is the sun is not always shinning…so a back up system is needed. . . more $. 5. Showers vs. baths / wash hands in cold water / full loads of laundry and dishwasher /…