Heat and Internal Energy Internal Energy U is





- Slides: 24

Heat and Internal Energy • Internal Energy U is the total energy associated with the microscopic components of the system – Includes kinetic and potential energy associated with the random translational, rotational and vibrational motion of the atoms or molecules – Also includes the intermolecular potential energy – Does not include macroscopic kinetic energy or external potential energy • Heat refers to the transfer of energy between a system and its environment due to a temperature difference between them – Amount of energy transferred by heat designated by symbol Q – A system does not have heat, just like it does not have work (heat and work speak to transfer of energy)

Units of Heat • The historical unit of heat was the calorie – A calorie is the amount of energy necessary to raise the temperature of 1 g of water from 14. 5°C to 15. 5°C – A Calorie (food calorie, with a capital C) is 1000 cal • Since heat (like work) is a measure of energy transfer, its SI unit is the joule – 1 cal = 4. 186 J (“Mechanical Equivalent of Heat”) – New definition of the calorie • The unit of heat in the U. S. customary system is the British thermal unit (BTU) – Defined as the amount of energy necessary to raise the temperature of 1 lb of water from 63°F to 64°F

More About Heat • Heat is a microscopic form of energy transfer involving large numbers of particles • Energy exchange occurs due to individual interactions of the particles – No macroscopic displacements or forces involved • Heat flow is from a system at higher temperature to one at lower temperature – Flow of heat tends to equalize average microscopic kinetic energy of molecules • When 2 systems are in thermal equilibrium, they are at the same temperature and there is no net heat flow • Energy transferred by heat does not always mean there is a temperature change (see phase changes)

Heat Transfer Simulation presented in class. (Activ. Physics Online Exercise #8. 6, copyright Addison Wesley publishing)

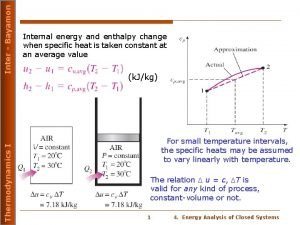
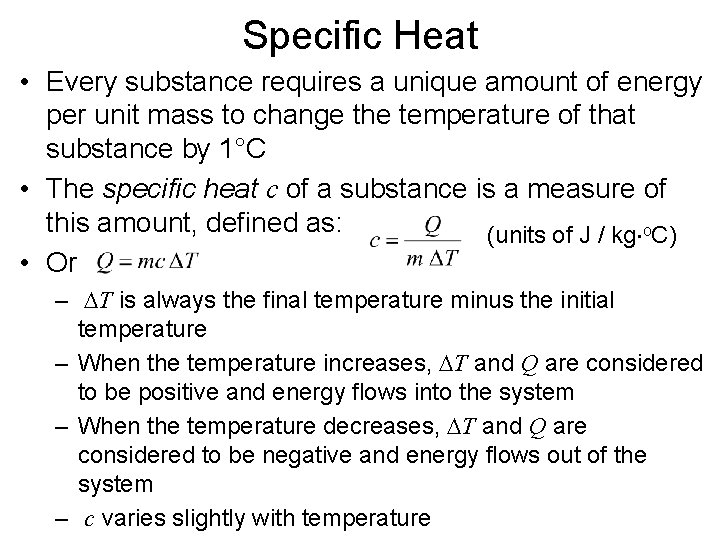
Specific Heat • Every substance requires a unique amount of energy per unit mass to change the temperature of that substance by 1°C • The specific heat c of a substance is a measure of this amount, defined as: (units of J / kg o. C) • Or – DT is always the final temperature minus the initial temperature – When the temperature increases, DT and Q are considered to be positive and energy flows into the system – When the temperature decreases, DT and Q are considered to be negative and energy flows out of the system – c varies slightly with temperature

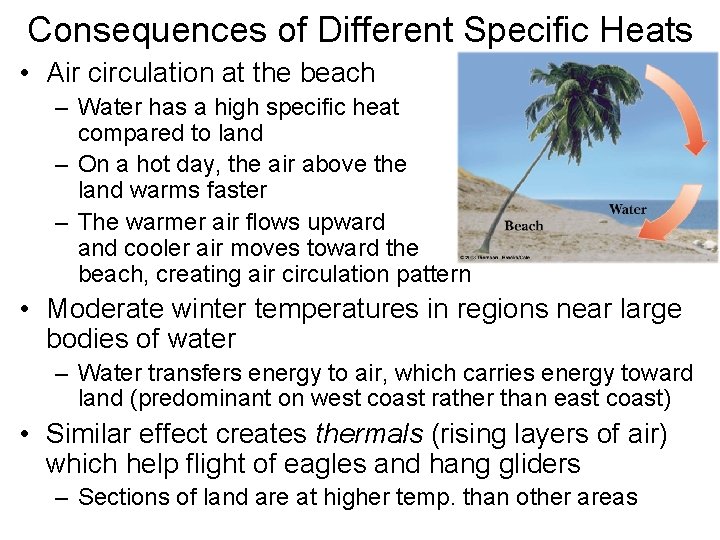
Consequences of Different Specific Heats • Air circulation at the beach – Water has a high specific heat compared to land – On a hot day, the air above the land warms faster – The warmer air flows upward and cooler air moves toward the beach, creating air circulation pattern • Moderate winter temperatures in regions near large bodies of water – Water transfers energy to air, which carries energy toward land (predominant on west coast rather than east coast) • Similar effect creates thermals (rising layers of air) which help flight of eagles and hang gliders – Sections of land are at higher temp. than other areas

Calorimetry • Calorimetry means “measuring heat” – In practice, it is a technique used to measure specific heat • Technique involves: – Raising temperature of object(s) to some value – Place object(s) in vessel containing cold water of known mass and temperature – Measure temperature of object(s) + water after equilibrium is reached • A calorimeter is a vessel providing good insulation that allows a thermal equilibrium to be achieved between substances without any energy loss to the environment (styrofoam cup or thermos with lid) • Conservation of energy requires that: (Q > 0 (< 0) when energy is gained (lost))

Example Problem #11. 17 An aluminum cup contains 225 g of water and a 40 -g copper stirrer, all at 27°C. A 400 -g sample of silver at an initial temperature of 87°C is placed in the water. The stirrer is used to stir the mixture until it reaches its final equilibrium temperature of 32°C. Calculate the mass of the aluminum cup. Solution (details given in class): 80 g

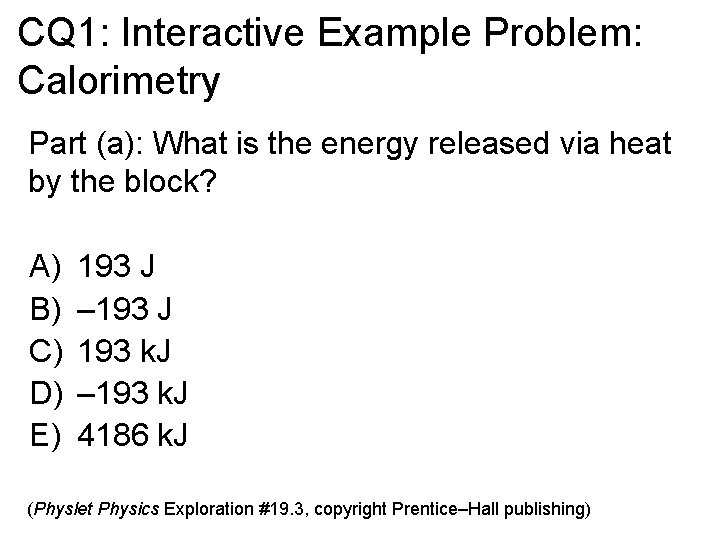
CQ 1: Interactive Example Problem: Calorimetry Part (a): What is the energy released via heat by the block? A) B) C) D) E) 193 J – 193 J 193 k. J – 193 k. J 4186 k. J (Physlet Physics Exploration #19. 3, copyright Prentice–Hall publishing)

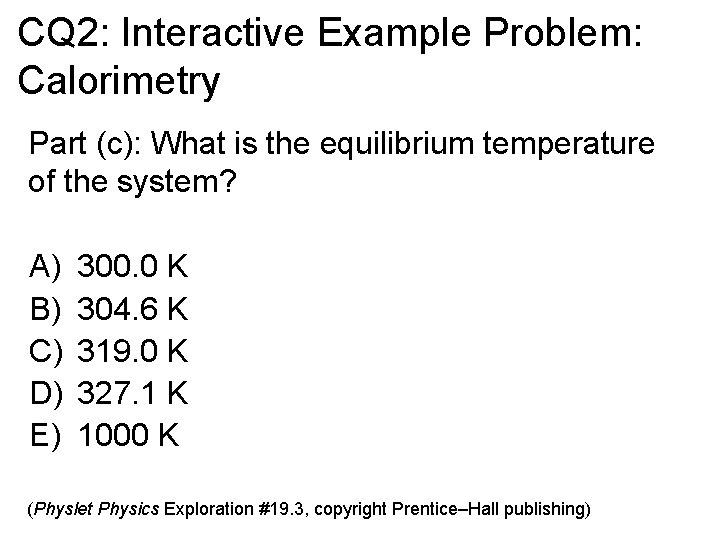
CQ 2: Interactive Example Problem: Calorimetry Part (c): What is the equilibrium temperature of the system? A) B) C) D) E) 300. 0 K 304. 6 K 319. 0 K 327. 1 K 1000 K (Physlet Physics Exploration #19. 3, copyright Prentice–Hall publishing)

Phase Transitions • A phase transition occurs when the physical characteristics of the substance change from one form to another • Common phase transitions are – Solid liquid (melting) – Liquid gas (boiling) • Phase transitions involve a change in the internal energy, but no change in temperature – Kinetic energy of molecules (which is related to temperature) is not changing, but their potential energy changes as work is done to change their positions • Energy required to change the phase of a given mass m of a pure substance is: – L = latent heat – depends on substance and nature of phase transition – + (–) sign used if energy is added (removed)

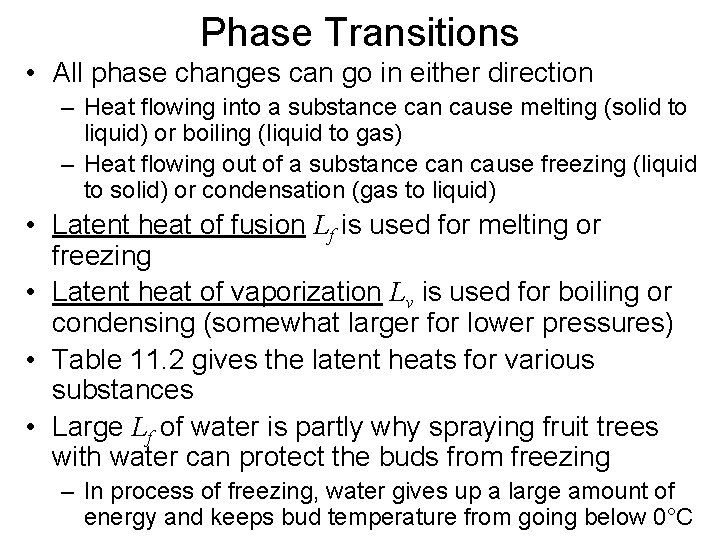
Phase Transitions • All phase changes can go in either direction – Heat flowing into a substance can cause melting (solid to liquid) or boiling (liquid to gas) – Heat flowing out of a substance can cause freezing (liquid to solid) or condensation (gas to liquid) • Latent heat of fusion Lf is used for melting or freezing • Latent heat of vaporization Lv is used for boiling or condensing (somewhat larger for lower pressures) • Table 11. 2 gives the latent heats for various substances • Large Lf of water is partly why spraying fruit trees with water can protect the buds from freezing – In process of freezing, water gives up a large amount of energy and keeps bud temperature from going below 0°C

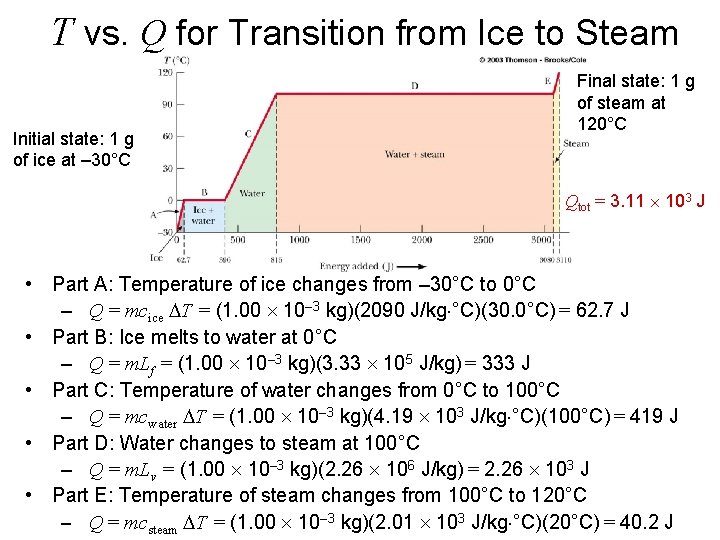
T vs. Q for Transition from Ice to Steam Initial state: 1 g of ice at – 30°C Final state: 1 g of steam at 120°C Qtot = 3. 11 103 J • Part A: Temperature of ice changes from – 30°C to 0°C – Q = mcice DT = (1. 00 10– 3 kg)(2090 J/kg °C)(30. 0°C) = 62. 7 J • Part B: Ice melts to water at 0°C – Q = m. Lf = (1. 00 10– 3 kg)(3. 33 105 J/kg) = 333 J • Part C: Temperature of water changes from 0°C to 100°C – Q = mcwater DT = (1. 00 10– 3 kg)(4. 19 103 J/kg °C)(100°C) = 419 J • Part D: Water changes to steam at 100°C – Q = m. Lv = (1. 00 10– 3 kg)(2. 26 106 J/kg) = 2. 26 103 J • Part E: Temperature of steam changes from 100°C to 120°C – Q = mcsteam DT = (1. 00 10– 3 kg)(2. 01 103 J/kg °C)(20°C) = 40. 2 J

Evaporation and Condensation • The previous example shows why a burn caused by 100°C steam is much more severe than a burn caused by 100°C water – Steam releases large amount of energy through heat as it condenses to form water on the skin – Much more energy is transferred to the skin than would be the case for same amount of water at 100°C • Evaporation is similar to boiling – Molecular bonds are being broken by the most energetic molecules – Average kinetic energy is lowered as a result, which is why evaporation is a cooling process – Approximately the same latent heat of vaporization applies – Reason why you feel cool after stepping out from a swimming pool

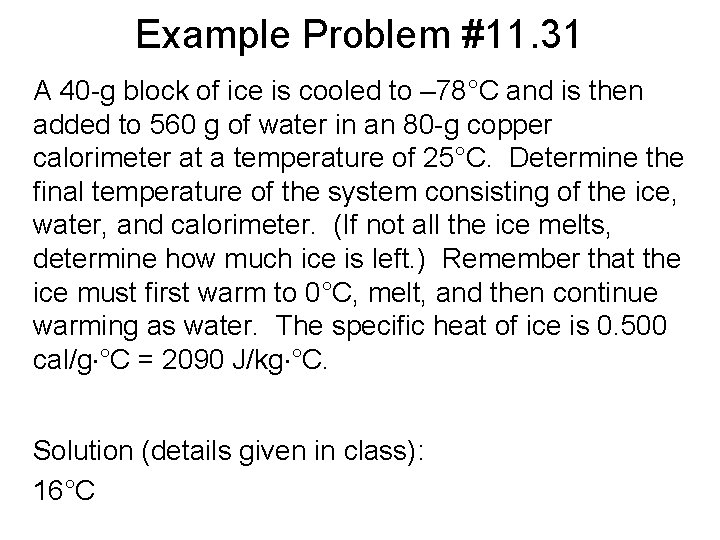
Example Problem #11. 31 A 40 -g block of ice is cooled to – 78°C and is then added to 560 g of water in an 80 -g copper calorimeter at a temperature of 25°C. Determine the final temperature of the system consisting of the ice, water, and calorimeter. (If not all the ice melts, determine how much ice is left. ) Remember that the ice must first warm to 0°C, melt, and then continue warming as water. The specific heat of ice is 0. 500 cal/g °C = 2090 J/kg °C. Solution (details given in class): 16°C

Conduction • Energy can be transferred via heat in one of three ways: conduction, convection, radiation • Conduction occurs with temperature differences • Transfer by conduction can be understood on an atomic scale – It is an exchange of energy between microscopic particles by collisions – Less energetic particles gain energy during collisions with more energetic particles – Net result is heat flow from higher temperature region to lower temperature region • Rate of conduction depends upon the characteristics of the substance – Metals are good conductors due to loosely-bound electrons

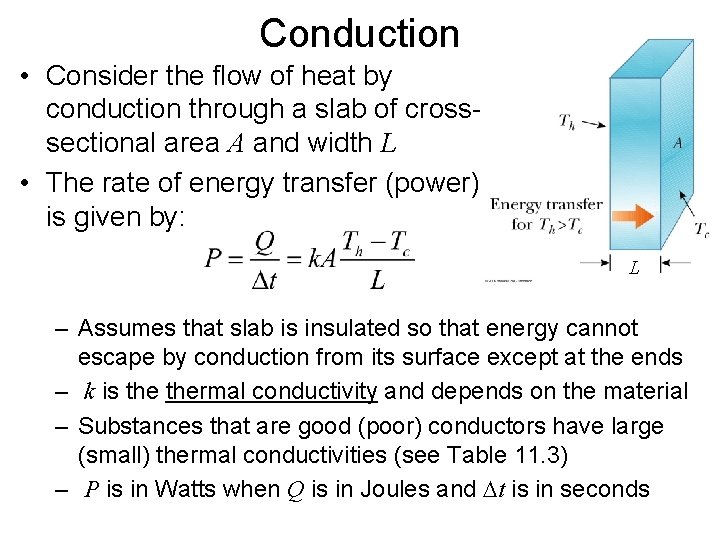
Conduction • Consider the flow of heat by conduction through a slab of crosssectional area A and width L • The rate of energy transfer (power) is given by: L – Assumes that slab is insulated so that energy cannot escape by conduction from its surface except at the ends – k is thermal conductivity and depends on the material – Substances that are good (poor) conductors have large (small) thermal conductivities (see Table 11. 3) – P is in Watts when Q is in Joules and Dt is in seconds

Home Insulation • In engineering, the insulating quality of materials are rated according to their R value: R = L / k • R values have strange units: °F ft 2 / (Btu/h) – That’s why units are not usually given! • Substances with larger R value are better insulators • For multiple layers, the total R value is the sum of the R values of each layer • Still air provides good insulation, but moving air increases the energy loss by conduction in a home – Much of thermal resistance of a window is due to the stagnant air layers rather than to the glass

Convection • Convection is heat flow by the movement of a fluid • When the movement results from differences in density, it is called natural convection (fluid currents are due to gravity) – Air currents at the beach – Water currents in a saucepan while heating • When the movement is forced by a fan or a pump, it is called forced convection (fluid is pushed around by mechanical means – fan or pump) – Forced-air heating systems – Hot-water baseboard heating – Blood circulation in the body (although air currents move under natural convection)

Thermal Radiation • Thermal radiation transfers energy through emission of electromagnetic waves – does not require physical contact • All objects radiate energy continuously in the form of electromagnetic waves due to thermal vibrations of the molecules – At ordinary temperatures (~20°C) nearly all the radiation is in the infrared (wavelengths longer than visible light) – At 800°C a body emits enough visible radiation to be selfluminous and appears “red-hot” – At 3000°C (incandescent lamp filament) the radiation contains enough visible light so the body appears “whitehot” • An ideal emitter and absorber of radiation is called a blackbody (would appear black)

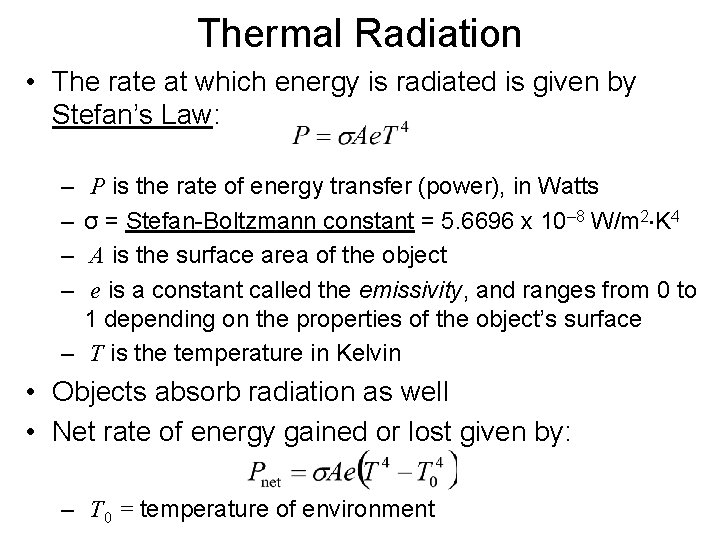
Thermal Radiation • The rate at which energy is radiated is given by Stefan’s Law: – – P is the rate of energy transfer (power), in Watts σ = Stefan-Boltzmann constant = 5. 6696 x 10– 8 W/m 2 K 4 A is the surface area of the object e is a constant called the emissivity, and ranges from 0 to 1 depending on the properties of the object’s surface – T is the temperature in Kelvin • Objects absorb radiation as well • Net rate of energy gained or lost given by: – T 0 = temperature of environment

Applications of Thermal Radiation • Choice of clothing – Black fabric acts as a good absorber, so about half of the emitted energy radiates toward the body – White fabric reflects thermal radiation well • Thermography as medical diagnostic tool – Measurement of emitted thermal energy using infrared detectors, producing a visual display (see Fig. 11. 13) – Areas of high temperature are indicated, showing regions of abnormal cellular activity • Measuring body temperature – Radiation thermometer measures the intensity of the infrared radiation from the eardrum (see Fig. 11. 14) – Eardrum is good location to measure temperature since it is near hypothalamus (body’s temperature control center)

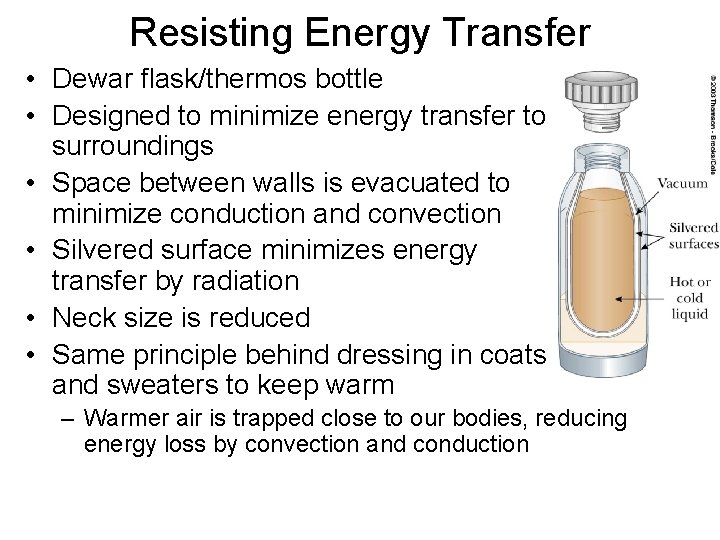
Resisting Energy Transfer • Dewar flask/thermos bottle • Designed to minimize energy transfer to surroundings • Space between walls is evacuated to minimize conduction and convection • Silvered surface minimizes energy transfer by radiation • Neck size is reduced • Same principle behind dressing in coats and sweaters to keep warm – Warmer air is trapped close to our bodies, reducing energy loss by convection and conduction

Global Warming • Analogous to a greenhouse – Visible light and short-wavelength infrared radiation are absorbed by contents of greenhouse, resulting in the emission of longer-wavelength infrared radiation (IR) – Longer-wavelength IR absorbed by glass – Glass emits IR, half of which is emitted back inside the greenhouse – Convection currents are inhibited by the glass (although this is not mirrored in Earth’s atmosphere) • Earth’s atmosphere fills role of glass roof in greenhouse – “Greenhouse gasses” like CO 2 are particularly good absorbers of IR – More greenhouse gasses in the atmosphere means more IR is absorbed and Earth’s surface becomes warmer
 Heat and internal energy
Heat and internal energy Specific latent heat of fusion formula
Specific latent heat of fusion formula Thermal energy vs heat energy
Thermal energy vs heat energy Thermal capacity
Thermal capacity Dry heat example
Dry heat example Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Elements of internal control system
Elements of internal control system Vouching in auditing
Vouching in auditing Tube side exchanger hydro testing
Tube side exchanger hydro testing Dtfd switch
Dtfd switch What is the difference between thermal energy and heat?
What is the difference between thermal energy and heat? Specific heat capacity of lead j/kg c
Specific heat capacity of lead j/kg c Difference between heat and thermal energy
Difference between heat and thermal energy Thermal energy vs heat
Thermal energy vs heat What is the difference between thermal energy and heat?
What is the difference between thermal energy and heat? Solar energy is radiant light and heat from the sun
Solar energy is radiant light and heat from the sun Heat thermal energy and temperature
Heat thermal energy and temperature Heat flow
Heat flow How does sound travel
How does sound travel Relationship between heat and energy
Relationship between heat and energy Formula formula
Formula formula Chapter 16 thermal energy and heat
Chapter 16 thermal energy and heat What is cv in thermodynamics
What is cv in thermodynamics Relation between partition function and internal energy
Relation between partition function and internal energy