Heat and Heat Transfer Scales of Measurement Celsius









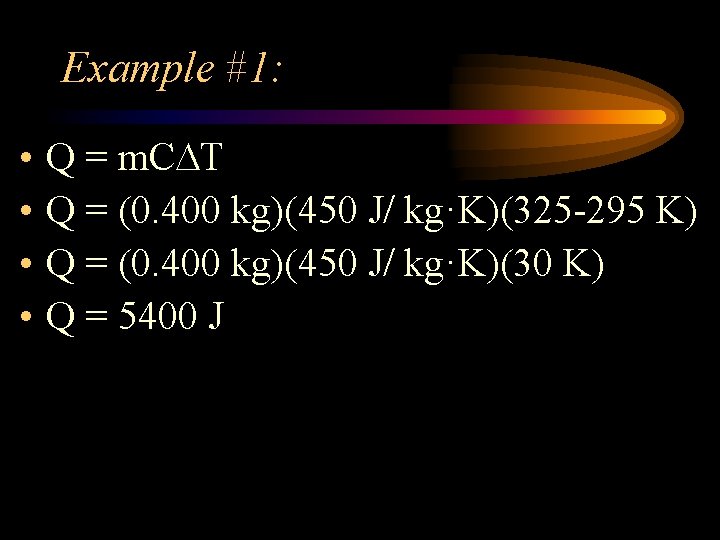


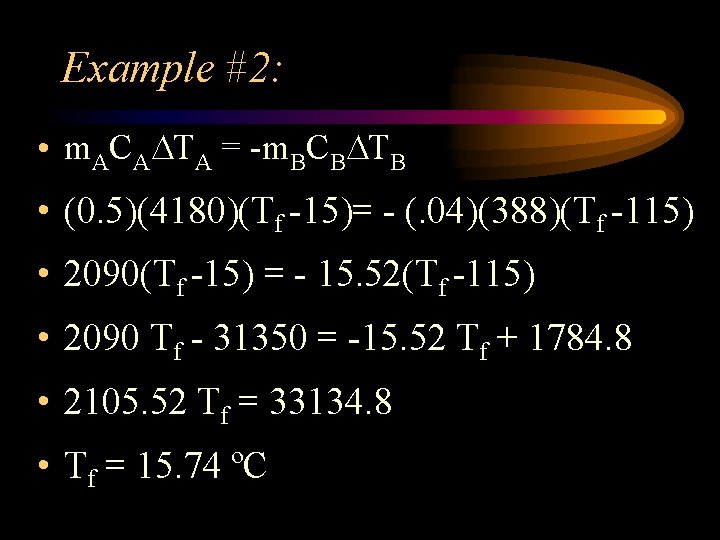
- Slides: 19

Heat and Heat Transfer

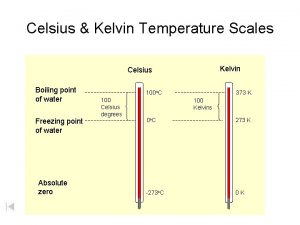
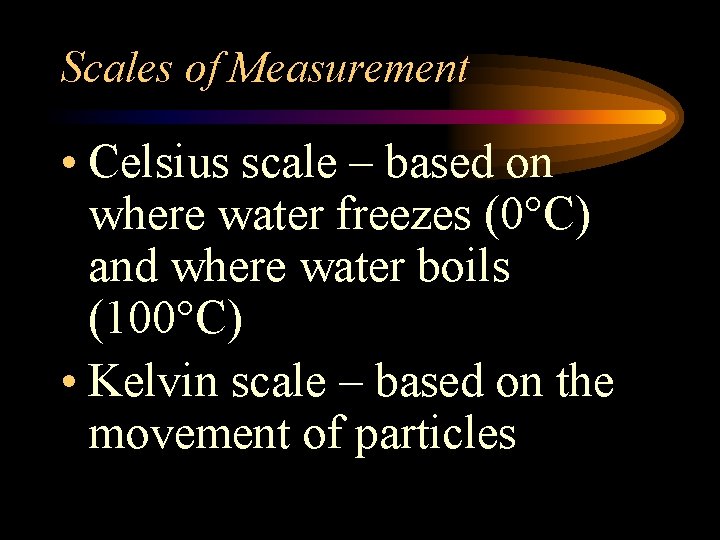
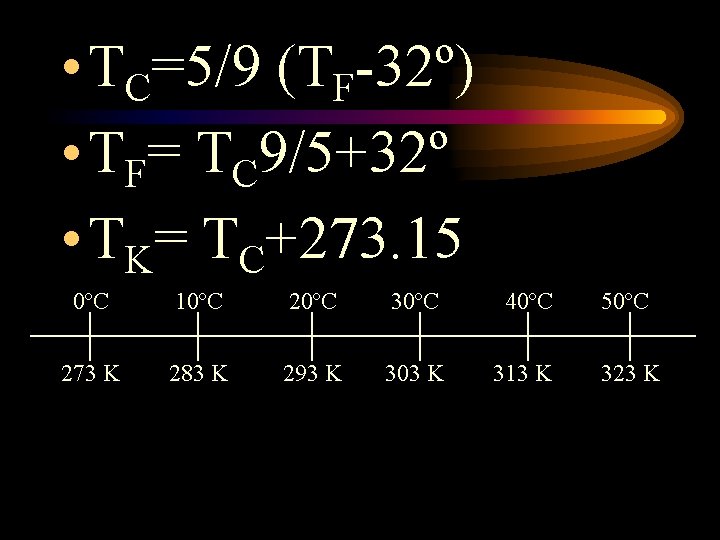
Scales of Measurement • Celsius scale – based on where water freezes (0 C) and where water boils (100 C) • Kelvin scale – based on the movement of particles

Absolute Zero • At 0 K, all particle movement has ceased • It is impossible to have a temperature lower than 0 K • 0 K = -273 C

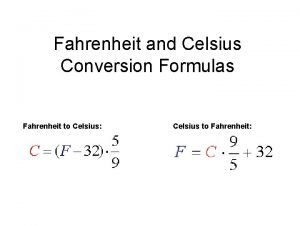
• TC=5/9 (TF-32º) • TF= TC 9/5+32º • TK= TC+273. 15 0ºC 10ºC 20ºC 30ºC 40ºC 50ºC 273 K 283 K 293 K 303 K 313 K 323 K

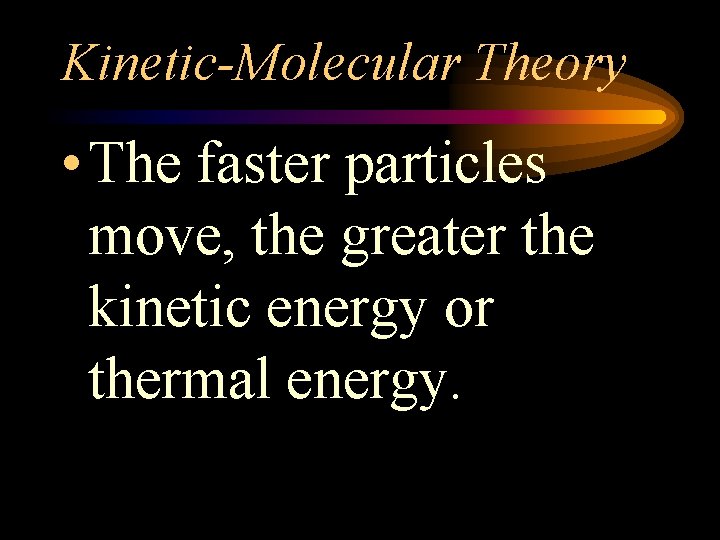
Kinetic-Molecular Theory • The faster particles move, the greater the kinetic energy or thermal energy.

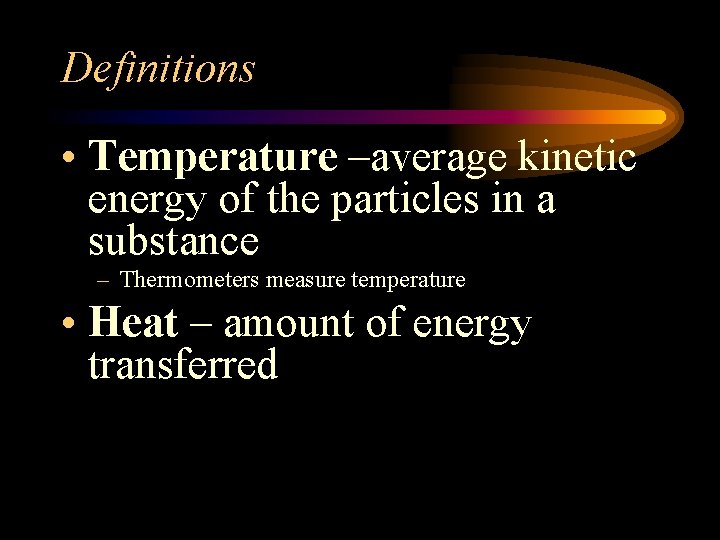
Definitions • Temperature –average kinetic energy of the particles in a substance – Thermometers measure temperature • Heat – amount of energy transferred

Thermal equilibrium • Energy will always travel from an area of higher energy to an area of lower energy.

Thermal Equilibrium, cont. • Two substances with different energies transfer energy (higher lower) until their energies are equal. • This point is “thermal equilibrium”.

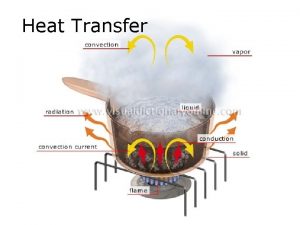
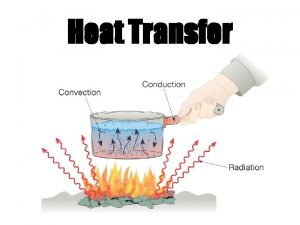
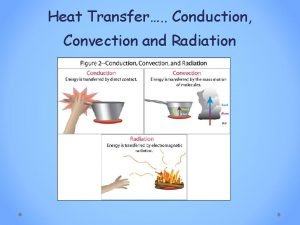
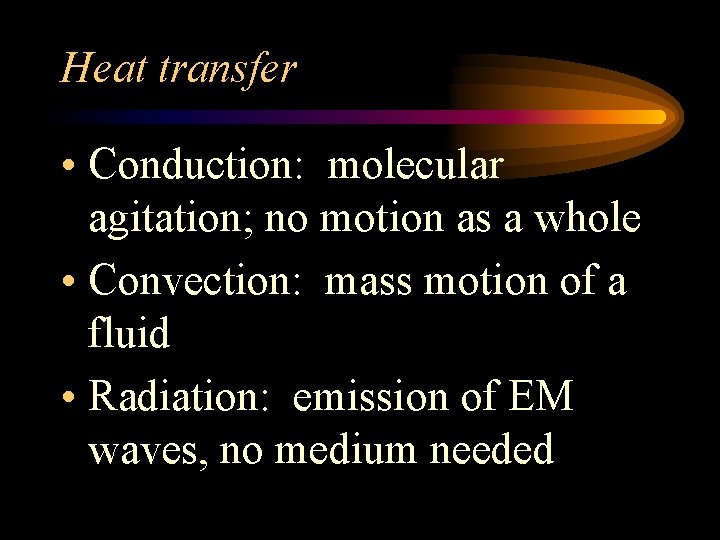
Heat transfer • Conduction: molecular agitation; no motion as a whole • Convection: mass motion of a fluid • Radiation: emission of EM waves, no medium needed

Conduction • As materials are heated, electrons gain thermal energy which means they move faster. • As the electrons in a substance collide, the energy is transferred to surrounding electrons. • The actual molecules do not change places.

Convection • Heating occurs due to the motion of a fluid. • When a fluid is heated, it becomes less dense and rises. The cooler air is more dense and circulates to the bottom where it is heated and begins the process again.

Radiation • Radiation does not require a medium to transmit energy. This type of energy is called radiant energy and it travels in electromagnetic waves. • High temperatures emit short wavelengths whereas low temperatures emit long wavelengths.

Specific Heat • Amount of energy that must be added to the material to raise the temperature of a unit mass one temperature unit. • The units of specific heat are J/kg·K or J/kg·°C

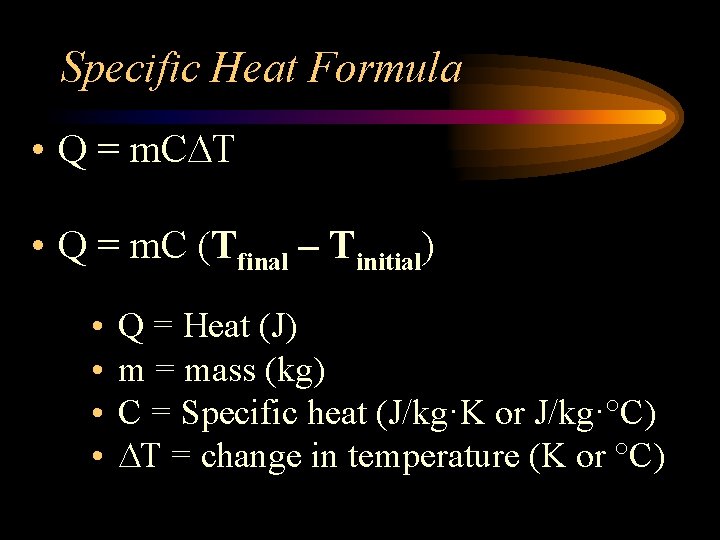
Specific Heat Formula • Q = m. C T • Q = m. C (Tfinal – Tinitial) • • Q = Heat (J) m = mass (kg) C = Specific heat (J/kg·K or J/kg·°C) T = change in temperature (K or °C)

Example #1: • A 0. 400 kg block of iron is heated from 295 K to 325 K. How much heat had to be transferred to the iron if the specific heat of iron is 450 J/ kg·K?
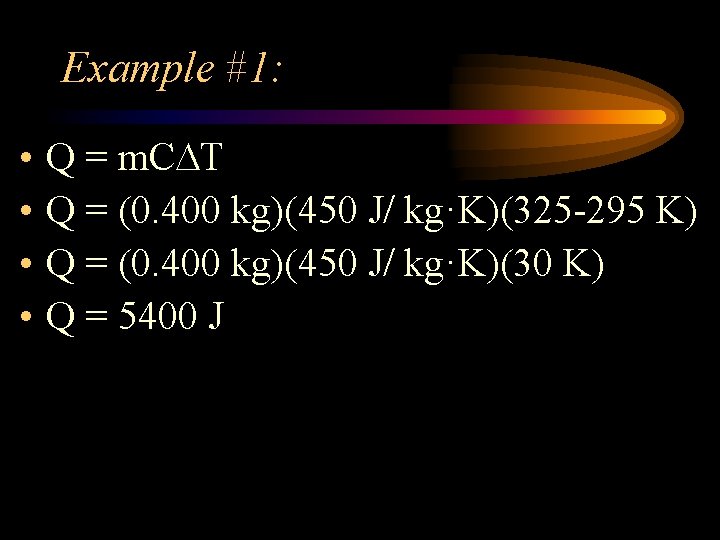
Example #1: • • Q = m. C T Q = (0. 400 kg)(450 J/ kg·K)(325 -295 K) Q = (0. 400 kg)(450 J/ kg·K)(30 K) Q = 5400 J

Law of Conservation of Energy • Energy lost by one object must be equal to the amount gained by another object. • Energy lost = - Energy gained • m. ACA TA = -m. BCB TB

Example #2: • A container has 0. 50 kg of water at 15 C. A 0. 040 kg block of zinc at 115 C is placed in the water. What is the final temperature of the system? (Czinc = 388 J/kg· C and Cwater = 4180 J/kg· C)
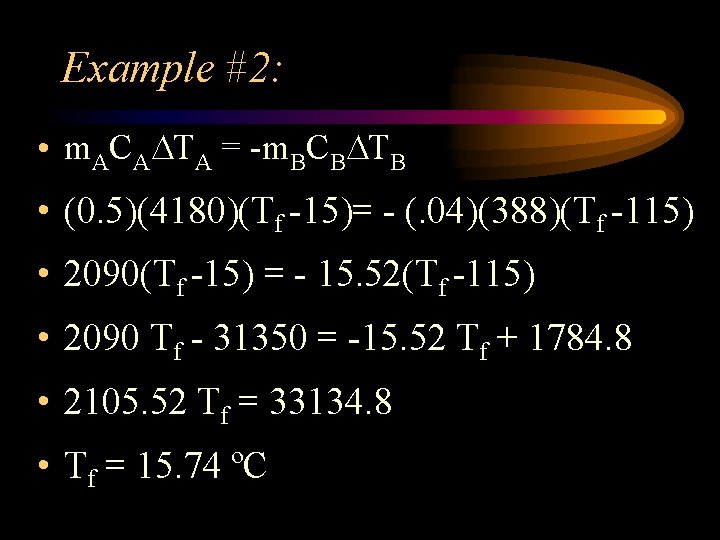
Example #2: • m. ACA TA = -m. BCB TB • (0. 5)(4180)(Tf -15)= - (. 04)(388)(Tf -115) • 2090(Tf -15) = - 15. 52(Tf -115) • 2090 Tf - 31350 = -15. 52 Tf + 1784. 8 • 2105. 52 Tf = 33134. 8 • Tf = 15. 74 ºC
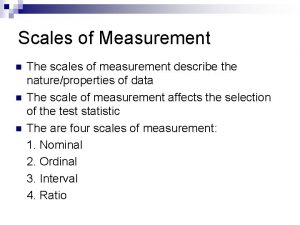
 What are the 4 measurement scales?
What are the 4 measurement scales? Measurement scales
Measurement scales Measurement and scaling
Measurement and scaling Types of scales in educational measurement
Types of scales in educational measurement Measurement scales in research
Measurement scales in research Scale data
Scale data Measurement scales in research
Measurement scales in research Primary scales of measurement in marketing research
Primary scales of measurement in marketing research Types of measurement scales
Types of measurement scales Marketing measurement scales
Marketing measurement scales Magnitude is the property of “moreness”.
Magnitude is the property of “moreness”. 4 measurement scales
4 measurement scales 4 measurement scales
4 measurement scales Specific heat unit
Specific heat unit Heat transfer
Heat transfer Is a disturbance that transfers energy
Is a disturbance that transfers energy What is heat transfer conduction convection and radiation
What is heat transfer conduction convection and radiation Example of heat transfer by radiation
Example of heat transfer by radiation Entropy and heat transfer
Entropy and heat transfer Heat and mass transfer cengel 4th edition pdf
Heat and mass transfer cengel 4th edition pdf