Heat and Calorimetry Energy Absorbed SOLID LIQUID Energy

Heat and Calorimetry Energy Absorbed SOLID LIQUID Energy Released GAS

Heat = “transfer” heat of thermal energy It’s incorrect to say matter “contains” heat. 80 o. C 20 o. C Matter “contains” KE and PE. WS 1 Energy of Motion Stored Energy (moving or vibrating) Thermal Energy (bonds & attractions) Chemical Energy
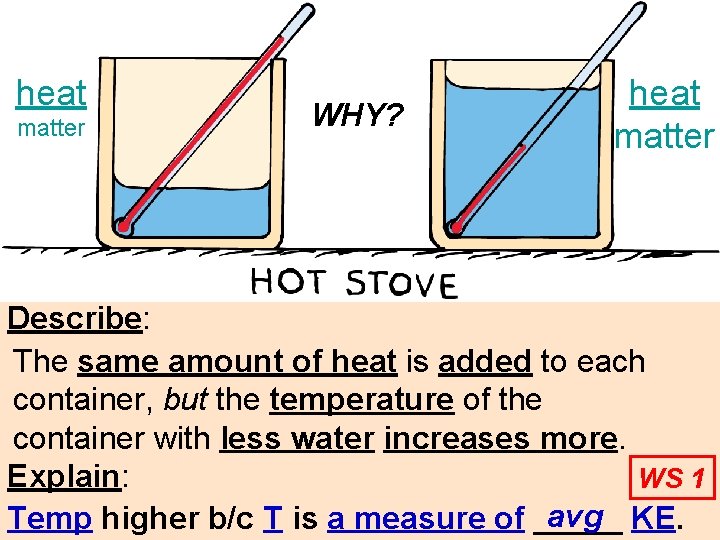
heat matter WHY? heat matter Describe: The same amount of heat is added to each container, but the temperature of the container with less water increases more. Explain: WS 1 avg KE. Temp higher b/c T is a measure of _____

specific heat capacity(c): heat to change WS 1 1 gram by 1 o. C Different substances have different capacities for storing energy. + 4. 18 J of heat c (water) = o. C 4. 18 J/g ____ The water in food holds 4 x the energy as an aluminum pan.
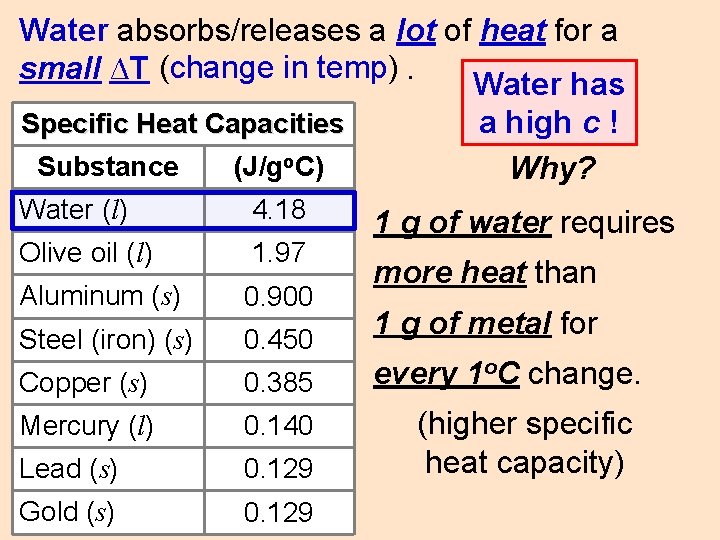
Water absorbs/releases a lot of heat for a small ∆T (change in temp). Water has Specific Heat Capacities Substance (J/go. C) Water (l) 4. 18 Olive oil (l) 1. 97 Aluminum (s) 0. 900 Steel (iron) (s) 0. 450 Copper (s) 0. 385 Mercury (l) 0. 140 Lead (s) 0. 129 Gold (s) 0. 129 a high c ! Why? 1 g of water requires more heat than 1 g of metal for every 1 o. C change. (higher specific heat capacity)

Substance (J/go. C) Water (l) (demo) 4. 18 Aluminum (s) 0. 900 Al metal atoms vibrate in place. Water “soaks up” (stores) a lot of energy in intramolecular movements and water has intermolecular attractions (H-bonds) a high c ! without changing average KE (Temp) vibrating stretching rotating

Heat transferred can be measured and calculated. Calorimetry q = mc T given on exam WS 1 q = heat (J) m = mass (g) c = specific heat (J/go. C) (4. 18 J/go. C for water) ∆T = change in T (Tfinal – Tinitial)

Sample Calculation: How much heat is needed to warm 500 g of water from 25 o. C to 100 o. C? q = mc T c = 4. 18 J/go. C q = (500)(4. 18)(100 – 25) q = 157, 000 J

Quick Quiz! 1. Temperature is directly proportional to the _____ of a substance. A. thermal energy B. vibrational kinetic energy C. average kinetic energy D. total kinetic energy

Quick Quiz. 2. Heat is simply another word for _____. A. temperature B. internal energy C. energy transferred from high to low temp D. thermal energy transferred

Quick Quiz. 3. How much energy would it take to raise 1 gram of liquid water from 20 o. C to 30 o. C? A. 4. 18 J B. 20. 9 J C. 41. 8 J D. 209 J + 4. 18 J

Quick Quiz. 4. How many joules would it take to raise 10 grams of liquid water from 20 o. C to 30 o. C? A. 2090 J B. 418 J C. 209 J D. 41. 8 J + 4. 18 J
- Slides: 12